Chapter 160
Vascular Trauma
Military
Todd E. Rasmussen, Charles J. Fox
The views expressed in this chapter are those of the authors and do not reflect the official policy of the Department of Defense, the United States Army or Air Force, or other departments of the United States government.
Vascular injury in the military has special significance. Combat-related injuries to major vessels present unique technical challenges and result in hemorrhage that is responsible for 90% of potentially preventable deaths on the battlefield.1,2 The most lethal injuries are those that cause disruption to vascular structures in the torso, which includes the chest and abdomen.3,4 Torso injury is reportedly the cause of half of potentially survivable hemorrhagic deaths, followed by extremity vascular injury, which is responsible for one third.1 The front lines of a battleground are chaotic, located in harsh environments, and vary widely, depending on the goal and scope of the military operation. Surgical care may be rendered in tents or buildings of opportunity that lack suitable light and ventilation or in more established and well-equipped forward surgical hospitals. These austere conditions, combined with the technical demands associated with the treatment of vascular injury, necessitate the early and deliberate preparation of military medics and surgeons at all levels of care to ensure the successful management of vascular trauma.2
Historic Advances Through Military Conflict
Contributions from the armed conflicts of the 20th century have defined the standards for vessel ligation or repair of arterial and venous injuries in resource-limited situations. Since the Vietnam War, there has been considerable modernization of the battlefield medical environment, which has translated into a measurable survival advantage.2,5 Forward surgical capability, expeditious evacuation, and new and effective resuscitation strategies have provided the foundation for innovation and progress. Lessons learned during current U.S. military operations continue to advance the practice of vascular trauma surgery, and these techniques are directly translated to surgical practices in trauma centers around the world.
World War II
A classic paper by DeBakey et al illustrated the challenges of vessel repair during wartime, revealing only 81 repairs of 2471 arterial injuries during World War II.6 Saphenous bypass grafts were impractical at the time, and all but three injuries were repaired by lateral suture. Amputation followed vessel ligation in nearly half of extremity vascular injuries during this era. Therefore, during World War II, evacuation delays, practical difficulties, and poor physiologic conditions led to the conclusion that vessel ligation, although not the “procedure of choice, is one of necessity.”6
Korean War
In 1952 during the Korean War, arterial repair of vascular injury was introduced by Frank Spencer and a team of U.S. Marine Corps medics; it originally consisted of a cadaveric femoral artery used as an interposition graft conduit in the injured extremity.7 A surgical research team based at Walter Reed Army Hospital and led by Captain John Howard was established to study these reported successes and other challenges associated with vascular injury in an effort to optimize management strategies.8 Several more comprehensive reports on successful arterial repair performed during the Korean War followed, including classic papers from Colonel Carl Hughes, which gained the attention of the Office of the Army Surgeon General.8–10 Hughes demonstrated an impressive reduction in the amputation rate among 269 repairs—from 49% in World War II to 13% during the Korean War. Despite the introduction of rotary wing casualty evacuation (CASEVAC) during the Korean War, significant time delays and resuscitation requirements remained the primary Achilles heel of successful vascular injury management.8
Vietnam War
Forward surgical capabilities and advances in CASEVAC continued to reduce ischemia time and commonly led to successful arterial reconstructions during the Vietnam War. The management of complex injuries, such as those involving the popliteal artery and vein or those associated with severe open contaminated fractures, became the focus of attention during this period.11–14 Under the guidance of Major Norman Rich, the Vietnam Vascular Registry provided details of the treatment of more than 1000 wartime vascular injuries and confirmed and extended the experience of Hughes and others in the Korean War. The Vietnam Vascular Registry now serves as a reference standard for the application of vascular surgery during the modern conflicts of the 21st century.15
Global War on Terror
For decades, the vascular injury experience of past wars was thought to be unapproachable with regard to the duration of conflict and the number of injuries. With more than 8000 deaths and approximately 50,000 combat-related injuries in more than a decade of modern warfare (http://www.icasualties.org), the Global War on Terror (GWOT) has proved to be a formidable and sustained military campaign. During this conflict, modern advances have allowed a concerted effort to reduce deaths from potentially survivable vascular injuries and to improve the quality of functional extremity salvage (i.e., saving life and limb).1
At the beginning of the GWOT, the Department of Defense implemented a testing, training, and fielding program for battlefield tourniquets.16–18 The effectiveness of early tourniquet application observed in Iraq and Afghanistan has led to doctrinal changes that have produced a surge of patients with vascular injuries who, in the past, would not have reached a field hospital alive (Fig. 160-1).19,20 Although current military endeavors regarding the appropriateness of tourniquet use began with trepidation, the forward deployment of surgical capabilities provides for limited tourniquet duration, thus increasing the effectiveness of tourniquets and reducing the rate of associated complications. In addition, the development of the Joint Theater Trauma System has improved surgical care and reduced mortality by implementing clinical practice guidelines and performing outcomes research emerging from the Joint Theater Trauma Registry. The GWOT Vascular Initiative is a comprehensive registry designed to study patterns of vessel injury and methods of vascular repair and to provide more complete long-term analysis of patient outcomes.
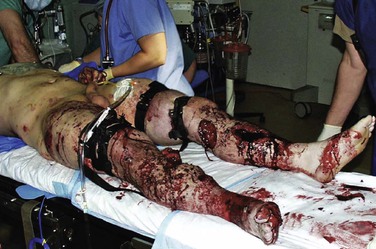
Figure 160-1 Patient with severe injuries to both lower extremities with two tourniquets on each leg. In such cases, the presence or absence of a vascular injury may not be determined until the tourniquets are released at the appropriate time and the patient is warmed and resuscitated. Note that the patient is in the operating room and that all the tourniquets remain in place while the anesthesia team tends to resuscitation needs. In this case, the tourniquets were released slowly in sequence, allowing the anesthesia team to treat any untoward effects of reperfusion. The patient is positioned on the table so that fluoroscopy can be performed during the operation to assess for fracture or so that arteriography can be performed.
Other modern advances include the routine use of personal protective gear (body armor), the deployment of level II facilities at more forward locations, and the selective application of surgical adjuncts (e.g., temporary vascular shunts, fasciotomies).21–26 Progress in the management of complex soft tissue wounds associated with vascular injury, such as closed negative pressure wound therapy, has been impressive; also impressive is the increased complexity of in-theater repair, which now includes tibia-level reconstruction for select patterns of injury.27 Last, the application of endovascular technologies to the diagnosis and treatment of certain types of vascular, pelvic, and solid organ war-related injuries has become more widespread and generally accepted as a mainstay of surgical care.28–30 In aggregate, modern-day endeavors rooted in the wartime efforts and academic accomplishments of the past make the topic of wartime vascular injury not just relevant but enthralling.
Epidemiology
Rates of Wartime Vascular Injury
In the Vietnam War, vascular injuries accounted for 2% to 3% of battle-related injuries.13 Published reports from the wars in Afghanistan and Iraq demonstrate that the rate of vascular injury is as high as 9% to 12% of battle-related injuries, making the recorded frequency approximately five times that reported in previous wars.21–26,31,32 The increased incidence of vascular injury may have several explanations, including an increased awareness of and attention to recording such injuries. Undoubtedly, the effectiveness of tourniquets and modern body armor and the strategic forward placement of surgical capabilities also allow the treatment of vascular injuries that would have been fatal in past wars.33 Regardless of the reason, the distinct increase in the rate of vascular injuries during modern war underscores the importance of training and the maintenance of competency within the military surgical ranks.
Demographics
Not surprisingly, the overwhelming majority of combat troops sustaining wartime vascular injuries are young (mean age, 23 years) and male (95%).21–26 Most commonly, this group of patients has no preexisting cardiovascular disease or morbidity. However, depending on the location of the military surgical facility and the concept of operations of the wartime setting, military surgeons may be faced with vascular injuries in patients at both ends of the age spectrum.27,34 The availability of surgical care to segments of the local civilian population at U.S. theater hospitals during the GWOT has provided an uncommon experience in the management of wartime vascular injuries in pediatric and elderly patients. Peck et al27 documented this experience in patients with extremity vascular injuries who were as young as 5 years and as old as 65 years. Reconstruction of vascular injuries is unique in children, given the technical challenges related to the repair of small vessels that are not yet fully developed. In addition, depending on age and stage of development, pediatric patients have a propensity for collateralization and better potential tolerance of axial vessel ligation compared with adults. This fact makes observation or even ligation of certain extremity vascular injuries a more prudent course of action than attempting a highly complex vascular intervention or reconstruction in certain pediatric patients.
Anatomic Patterns
Contemporary patterns of wartime injury are similar to historical reports, with extremity vascular injuries being most common (Fig. 160-2).21–26 An in-theater report by Clouse et al22 on nearly 350 vascular injuries among both U.S. forces and the local population showed that extremity injuries were most common, and the rate of extremity injury was higher among U.S. forces (81%) than among civilians (70%). In addition, there was a proportionate lower incidence in truncal vascular injuries among U.S. forces (4%) compared with the local population (13%; see Fig. 160-2). These figures suggest the effectiveness of modern body armor available to those in combat.
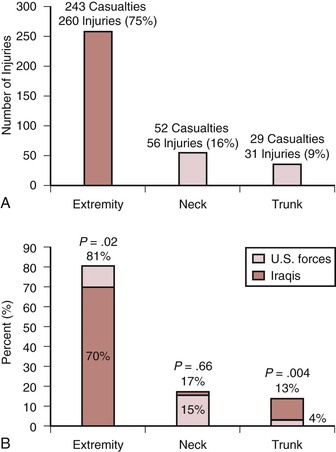
Figure 160-2 Distribution of vascular injuries at the 332nd Expeditionary Medical Group, Air Force theater hospital at Balad Air Base, Iraq, from September 1, 2004, through August 31, 2006. A, Distribution of vascular injuries by anatomic location (N = 347). B, Anatomic distribution of vascular injuries among U.S. forces and among the local population (N = 347). There are significant differences between these two groups in terms of the proportion of patients with extremity and trunk injuries, suggesting the efficacy of military body armor.
Lower extremity vascular injuries occur at approximately two times the rate of upper extremity injuries, reflecting the relative length of axial vessels and the exposed position of the lower extremity away from the protection of the torso. The anatomic distribution of arterial and venous injuries is roughly the same, although there is a slightly higher percentage of venous injuries in the neck and a lower percentage of upper extremity venous injuries (Tables 160-1 and 160-2).21,22 In the lower extremity, the superficial femoral artery is most commonly injured (33% to 37%), followed by the popliteal and tibial arteries (25% each). Injuries to the proximal common femoral artery and profunda femoris are less common because of their proximity to the protective structures of the torso and their lethality when they do occur. In a detailed analysis of penetrating femoropopliteal injuries during modern warfare, Woodward et al24 showed that nearly 50% of lower extremity vascular injuries had a combined arterial and venous component. Arterial injury in the neck constitutes roughly 15% of all arterial trauma and is equally distributed among the common, external, and internal carotid arteries and the vertebral artery, whereas all venous injuries in the neck are to the jugular vein (see Tables 160-1 and 160-2).22
Table 160-1
Location of Arterial Injuries in Military Trauma*
| Artery | Number | % |
| NECK | 42 | 14.0 |
| Common carotid | 13 | 4.3 |
| Internal carotid | 11 | 3.7 |
| External carotid | 9 | 3.0 |
| Vertebral | 9 | 3.0 |
| UPPER EXTREMITY | 76 | 25.3 |
| Subclavian-axillary | 11 | 3.7 |
| Brachial | 42 | 14.0 |
| Forearm | 23 | 7.6 |
| CHEST | 7 | 2.3 |
| Supra-aortic trunk | 4 | 1.3 |
| Thoracic aorta | 3 | 1.0 |
| ABDOMEN | 18 | 6.0 |
| Abdominal aorta | 5 | 1.7 |
| Renovisceral | 4 | 1.3 |
| Common iliac | 4 | 1.3 |
| Internal iliac | 2 | 0.7 |
| External iliac | 3 | 1.0 |
| LOWER EXTREMITY | 158 | 52.5 |
| Common femoral | 9 | 3.0 |
| Profunda femoris | 12 | 4.0 |
| Superficial femoral | 53 | 17.6 |
| Popliteal | 40 | 13.3 |
| Tibial | 44 | 14.6 |
| Total | 301 | 100.0 |
* Location of arterial injury by anatomic location and injured vessel in 301 arterial injuries during 24 months.
Data from Clouse WD, et al: In-theater management of vascular injury: 2 years of the Balad Vascular Registry. J Am Coll Surg 204:625-632, 2007.
Table 160-2
Location of Venous Injuries in Military Trauma*
| Vein | Number | % |
| NECK | 24 | 22.4 |
| Internal jugular | 24 | 22.4 |
| UPPER EXTREMITY | 9 | 8.4 |
| Subclavian-axillary | 9 | 8.4 |
| CHEST | 3 | 2.8 |
| Brachiocephalic | 2 | 1.9 |
| Inferior vena cava | 1 | 0.9 |
| ABDOMEN | 9 | 8.4 |
| Inferior vena cava | 4 | 3.7 |
| Common iliac | 2 | 1.9 |
| External iliac | 3 | 2.8 |
| LOWER EXTREMITY | 62 | 58.0 |
| Common femoral | 5 | 4.7 |
| Profunda femoris | 4 | 3.7 |
| Superficial femoral | 28 | 26.2 |
| Popliteal | 25 | 23.4 |
| Total | 107 | 100.0 |
* Location of venous injury by anatomic location and injured vessel in 107 venous injuries during 24 months.
Data from Clouse WD, et al: In-theater management of vascular injury: 2 years of the Balad Vascular Registry. J Am Coll Surg 204:625-632, 2007.
Because nearly all vascular injuries in wartime are caused by blast or high-velocity weaponry, approximately one third of those with vascular injuries have associated orthopedic injuries, and up to 20% have partial- or full-thickness burns23; the same percentage has an additional head or torso injury. All these associated injuries have an impact on the decision making related to the triage and ultimate treatment of vascular injury.
Mechanism of Injury
Penetrating mechanisms of injury are by far the most common, with explosive devices and gunshot wounds responsible for nearly all vascular injuries in wartime.6,8,13,21–23 In Operation Iraqi Freedom, improvised explosive devices were the cause of vascular injury in 55% of patients, and gunshot wounds accounted for 39% of injuries.21–23 Although the cause of vascular injury is often difficult to ascertain, and definitions of types of explosive devices may vary slightly, the proportion of gunshot wounds (25% to 45%) versus explosive devices (55% to 75%) responsible for vascular injuries has remained roughly the same in every conflict since World War II. In contrast to slight variations in the cause of vascular injury, which may vary by the operational setting (e.g., traditional versus asymmetrical), the anatomic distribution is largely constant, with extremity injuries always most prevalent.21–23
Levels of Care
The organization of surgical care in the theater of war requires the distribution of surgical capabilities to facilities and locations referred to as levels of care (Fig. 160-3).26,35 Each level of care (previously referred to as echelons) functions uniquely in the management of wartime vascular injury, attempting to prevent hemorrhage and to optimize functional outcome. There are five levels of care, ranging from level I (combat medic) to level V (facilities within the United States).35 The highest level of care in the area of responsibility, which encompasses the theaters of Iraq and Afghanistan, is level III surgical capability. Levels of care are part of an organizational pattern and may change according to the type of battlefield, the events on the ground, and the distribution of surgical expertise. The evacuation of injured troops through the various levels of care has specific terminology to allow uniform communication, tracking, and study of this step in the care of the wounded. The in-theater movement of casualties from the site of injury or from a level I location to a level II or III facility is referred to as casualty evacuation, or CASEVAC. In-theater movement between levels II and III facilities is termed medical evacuation, or MEDEVAC; evacuation out of theater is designated air evacuation, or AIREVAC (Fig. 160-4).
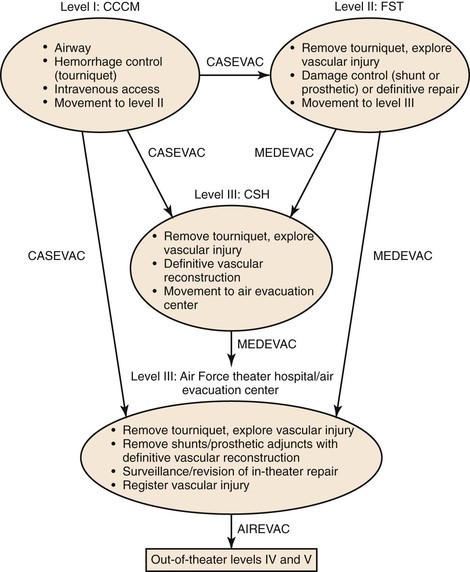
Figure 160-3 Evacuation scheme for injured troops through all five levels of care. Levels I, II, and III are in the theater of war. Level IV care is provided at Landstuhl Regional Medical Center in Germany. Level V represents tertiary military medical centers in the United States. AIREVAC, Air evacuation; CASEVAC, casualty evacuation; CCCM, combat casualty care medic; CSH, combat support hospital; FST, forward surgical team; MEDEVAC, medical evacuation.
Level I
Level I care is provided by the tactical combat casualty medic, who performs lifesaving measures and initiates movement of the wounded to treatment facilities such as Army Battalion Aid Stations or Marine Corps Shock Trauma Platoons. Level I care includes establishment of an airway; hemorrhage control, with or without application of a field dressing or tourniquet; and intravenous access. Because bleeding is the leading cause of potentially preventable death on the battlefield, the availability and use of certain tools to stop hemorrhage have become standardized among level I medics.16–18 The distribution of the Special Operations Forces Tactical Tourniquet (SOFTT) or the Combat Applications Tourniquet System (CATS) for hemorrhage control is one such change. Although improvised tourniquet devices have been used for hundreds of years, the more formal discussion of their safety and efficacy and the purchase of commercial devices have occurred since the experience with vascular injury in the Vietnam War.
SOFTT and CATS are designed to be placed with one hand and are carried by nearly all combat forces in Iraq and Afghanistan. Despite the potential harmful effects of inappropriate or prolonged application of tourniquets, experience with the use of tourniquets for hemorrhage control has been favorable.22–24,26 In rare cases, tourniquets have been applied unnecessarily or inappropriately; examples include application on extremities with isolated venous injuries or on extremities without injuries to major axial vessels. However, in these cases, the tourniquets were evaluated at level II or III facilities and removed in less than an hour, with few adverse effects.26 Preliminary data from the GWOT Vascular Initiative show that tourniquet use is documented in approximately one third of extremity vascular injuries.
In addition to the use of tourniquets, there has been a proliferation of commercially available topical hemostatic agents that can be applied by medics at the scene of the injury to assist with hemorrhage control. The two most common are HemCon (HemCon Medical Technologies Inc., Portland, Ore) and QuikClot (Z-Medica Corp., Wallingford, Ct).36–38 HemCon is a chitosan dressing that adheres strongly to soft tissues, sealing the wound site and concentrating red blood cells and clotting factors, including platelets. The active ingredient in QuikClot is zeolite granules, which avidly absorb water, concentrating red blood cells and clotting factors at the bleeding site in a significant exothermic reaction. In-theater experience with these agents is generally favorable, although each has potential complications and can make definitive vascular reconstruction more difficult.
Currently, the potential downside of using hemostatic adjuncts, topical agents, or tourniquets is accepted, knowing that the use of these tools will prevent death from hemorrhage in a percentage of wounded soldiers with select patterns of vascular injury.
Level II
In past military conflicts, capabilities at level II facilities were not well defined; however, in the decades since the Vietnam War, advances in the forward deployment of surgical resources have been formalized to provide a damage control capacity within minutes of injury. The GWOT represents the first prolonged military conflict in which this strategy and its impact on vascular injury have been tested. Each military service has a different level II unit with similar surgical capabilities. The Army deploys a level II medical treatment facility, a forward surgical team, or a combination of both. The Air Force deploys an expeditionary medical support unit, a mobile field surgical team, or a combination thereof. The Navy provides casualty receiving and treatment ships, and the Marine Corp has a forward resuscitative surgery system.34,39
The full impact of level II capability on vascular outcomes is unknown, although preliminary experience is favorable. In a report of the Marine Corps’ forward resuscitative surgical system, Chambers et al39 demonstrated that casualties were received at their level II facility within 30 minutes of injury. By comparison, during the Vietnam War, 85% of those with missile wounds underwent initial operation within 90 minutes of injury (at a level III facility, by today’s definitions).40 Woodward et al24 reported that during a 32-month period, 58 of 142 casualties (38%) with penetrating femoropopliteal injuries were seen at a level II facility before being evacuated to the Air Force theater hospital at Balad Air Base, a level III facility. This more forward level II capacity allows the earlier removal of tourniquets and more immediate identification and exploration of vascular injuries. This translates to earlier thrombectomy, application of heparin to the injured vessel, vascular reconstruction, placement of temporary shunts, and fasciotomy, all noted priorities in emergency wartime surgery.22–26,35,39
In contrast to the reported experience from the Vietnam Vascular Registry, a significant percentage of extremity vascular injuries are now managed with temporary vascular shunts to restore axial flow to the extremity.22–26,39 Currently, during periods of high influx of casualties, temporary vascular shunts are most commonly placed as damage control adjuncts at level II facilities as part of the triad of thrombectomy, restoration of flow, and fasciotomy.22,25,39
Data from the Balad Vascular Registry indicate that temporary vascular shunts have been used in 33% to 50% of extremity vascular injuries and that shunts placed in proximal injuries (e.g., femoropopliteal, axillosubclavian) are most effective. Patency of shunts in proximal vessels is greater than 90% when these injuries are re-explored at level III facilities after MEDEVAC.25 The patency rates of vascular shunts in wartime are achieved without the use of systemic heparinization, which reflects the preliminary large-animal data from Dawson et al.41 At level III facilities, the vascular injuries are re-explored, the shunts are removed, and definitive vascular reconstruction is performed. Anecdotal cases of successful arterial and venous shunting are not uncommon in injuries of the femoral vessels. Data from our group show that despite the poor patency of shunts placed in more distal vascular injuries (e.g., forearm, tibia), the early limb salvage rate in such cases is not compromised.25 Use of temporary vascular shunts in more distal vessels should be the exception and should be considered only when the procedure is technically straightforward, with exposed vessels and a good size match. In the case of most distal vascular injuries, ligation without shunting represents an important component of damage control. The extremity should then be assessed for perfusion with continuous wave Doppler examination. In most instances, the collateral circulation around such distal vascular injuries is sufficient to keep the extremity viable until a more complete evaluation can be performed. In these cases, the utility of continuous wave Doppler examination to demonstrate the presence or absence of even a weak arterial signal cannot be overemphasized.
Although there is appropriate concern about the use of shunts for long periods over extended evacuation distances, under the current system, patients treated at level II facilities arrive at level III facilities within 2 hours of injury (and often within 30 to 60 minutes).25,39 In a review of in-theater evacuation patterns, the average time from loading on a helicopter to arrival at a level III facility was 46 minutes.26 Rapid movement of patients with vascular shunts and effective communication between surgeons at both levels of care can increase the likelihood of shunt usefulness and minimize complications, such as shunt thrombosis or dislodgement. Currently, the use of temporary vascular shunts is encouraged in select patterns of proximal vascular injuries (including venous injuries) as part of the triad of early thrombectomy, restoration of flow, and fasciotomy.
Level III
Level III facilities in Iraq and Afghanistan include the Army’s combat support hospitals and the Air Force’s theater hospitals.26,35 Level III care is the highest or most comprehensive in any active combat theater. In light of the evacuation time to the level IV facility in Germany (8 to 12 hours from Iraq or Afghanistan), much of the operating historically performed at level IV facilities has now been pushed forward to level III locations in theater. This is especially true for vascular injuries, and the goal has been to place trained peripheral vascular surgeons at level III facilities. As part of this model, nearly all vascular injuries are definitively repaired before AIREVAC out of Iraq and Afghanistan, including the removal of all temporary shunts and the repair of extremity vascular injuries with autologous vein.26
Level III facilities are equipped with vascular instrument sets, continuous wave Doppler ultrasound, intraoperative fluoroscopy, blood banks, and intensive care units. In addition, there are accessories such as prosthetic graft material for aortic and great vessel repair, embolectomy catheters, thrombolytic agents, and removable vena cava filters. Recent reports from our group described the forward deployment of endovascular capabilities to a level III facility to provide an increasing number of critical interventions that can be offered by endovascular techniques when a suitable inventory exists.28 Examples include coil embolization of hemorrhage from pelvic fractures or select solid organ injuries, placement of covered stents in certain arterial injury patterns, and placement of temporary vena cava filters in patients unable to receive chemoprophylaxis because of associated solid organ or intracranial injuries.28 Removable filters are especially useful in the setting of concomitant pelvic or long bone fractures, which place patients who are unable to receive heparin at high risk for pulmonary embolus during extended AIREVAC.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



