Chapter 155
Vascular Trauma
Epidemiology and Natural History
Matthew J. Martin, William B. Long
Based on a chapter in the seventh edition by Raul Coimbra and David B. Hoyt
The epidemiology and natural history of most vascular diseases are related to underlying patient factors, disease processes, and severity and may be observed during prolonged periods. Vascular trauma is nearly the opposite and involves acute force injuries to usually normal and healthy vessels, with typically very short time intervals for diagnosis and intervention. Although the anatomy, exposure, and management principles are similar to those of elective vascular surgery, there is often not the luxury of time to perform a detailed and complete evaluation and preparation for treatment. The vascular trauma surgeon must proceed rapidly and definitively in the face of often incomplete and imperfect information, and thus the establishment of a solid foundation of knowledge about the epidemiology and natural history of vascular wounds is critical to the ultimate success or failure of treatment.
Historical Epidemiology and Evolution
The advances and developments in the treatment of vascular trauma, and in fact all aspects of trauma, are often described within the framework of major periods of conflict or war. The sheer volume and severity of injuries associated with combat present fertile ground for new observations, improvement or enhancement of known treatments, and development of completely novel medical and surgical techniques. However, the common characterization of this process as long periods of peacetime stasis punctuated by short and intense periods of wartime advancement is somewhat exaggerated and fails to appreciate many of the significant contributions made outside of the battlefield.
Major vascular trauma is most commonly associated with hemorrhage, and arguably the initial foundations of medical and surgical practice evolved around the control of bleeding. The mainstay for therapy of visible vascular injuries from the earliest medical history to the Renaissance (16th century) was the application of topical treatments, including poultices, bandages, styptics, and cautery.1 In 1552 Ambroise Paré, on exhausting his supply of boiling oil for topical treatment, performed a leg amputation successfully with vascular ligation. The obvious advantages in terms of both efficacy and patient comfort led to widespread adoption of ligatures as the standard of care for both arterial and major venous injuries for the next 400 years.
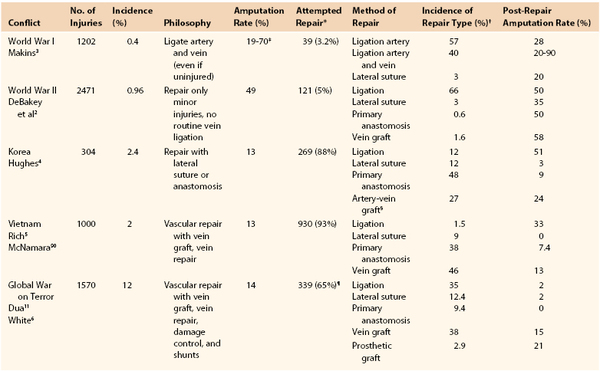
The 20th century ushered in a revolution in the field of vascular trauma and surgery as a result of multiple convergent factors, most important, the introduction of antisepsis, antibiotics, and safe anesthetic agents. The experiences reported from the major modern conflicts starting with World War I provide a highlight of the evolution and key milestones in vascular trauma management. Table 155-1 provides a summary of the reported wartime experiences and outcomes, including the overall and treatment-related amputation rates. Although the concepts of vascular repair and techniques for anastomoses or interposition grafts had been well described by the turn of the century, the lack of adequate equipment and training and the high incidence of complications prevented widespread acceptance.1 An additional key factor in determining candidacy for repair and particularly limb salvage is the time from injury to treatment, and average evacuation times in combat show a strong correlation with amputation rates (Fig. 155-1). Ligation of both arterial and venous injuries remained the standard of care through World War II, with repair attempted in only 3.2% (WWI) and 5% (WWII) of injuries.2 In addition, it was common practice in World War I to ligate not only the injured artery but also the associated uninjured vein in an attempt to “maintain within the limb for a longer period the smaller amount of blood supplied by the collateral arterial circulation.”3
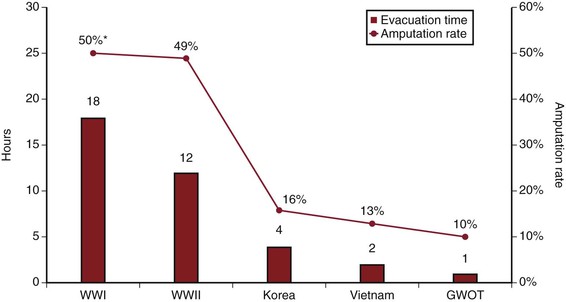
Figure 155-1 Trends in average time for evacuation of battlefield casualties to a forward medical facility (red bars) and the average all-cause amputation rates (red line) for major U.S. wars. GWOT, Global War on Terror.
A major shift can then be seen during the Korean War experience, with 88% of injuries undergoing an attempt at primary repair or anastomosis (60%) or interposition graft (27%).1,4 The Korean War experience is also notable for the widespread use of arterial homografts, which were later abandoned because of an increased failure and amputation rate (33%) compared with vein grafts (12%).4 During the Vietnam War, the recognition of the importance of careful data collection and analysis of long-term outcomes of combat vascular injuries led to the creation of the Vietnam Vascular Registry. Rich et al5 published landmark reports of the first 500 and 1000 patients, documenting the preference for attempted repair in the majority (93%) of injuries and describing longer term repair patency and amputation rates. The most modern reported combat epidemiologic data come from the decade-long conflicts in Iraq and Afghanistan, with several excellent series reporting both short-term and longer term outcomes. Hemorrhage has clearly been identified as the major source of mortality and morbidity at both the prehospital and in-hospital echelons on the modern battlefield. Of interest, the 12% incidence of vascular injuries is the highest ever reported during wartime and probably reflects improved detailed data collection as well as a shift in injury mechanism from conventional weaponry to the improvised explosive device.6 The predominance of the improvised explosive device and the improvements in body armor have resulted in an epidemiologic shift in injury patterns, with a high rate of extremity injury (53%) and decreased rate of major truncal injury (15%). The devastating nature of many of the extremity wounds and the inclusion of many distal arterial injuries probably explain the significant increase in vascular ligations (35%) compared with Vietnam and Korea.
Although historically major vascular injuries were relatively rare in the civilian setting, the development of machinery and motorized vehicles as well as the increase in urban violence and weaponry has resulted in an increased incidence of civilian vascular trauma. The exact incidence and distribution of injury mechanisms may vary widely between centers, depending on the setting (urban versus rural) and population served, but the overall reported incidence of civilian vascular injury has been similar to the historical combat experience at 1% to 4%. However, the reported incidence of major vascular injury is likely to be an underestimate and does not include patients who die at the trauma scene or before or immediately after hospital arrival. One analysis of autopsy reports of 552 trauma deaths identified major vessel disruption as the primary cause of death in 25% overall and in 26% of those with vital signs in the field.7 The majority of prehospital or immediate deaths from vessel disruption were due to aortic injury (55%), and most (78%) were associated with death within 15 minutes of injury.
Current Epidemiology of Vascular Injury
One of most important factors in understanding the epidemiology and patterns of vascular injuries and trauma is the collection of large samples with thorough and reliable data. This has traditionally been done by single centers performing chart review analyses during long periods or by analysis of wartime experience with limited follow-up data. Developments in computerized databases, automated data collection, and establishment of trauma systems with dedicated registrars have facilitated the collection and analysis of large and multicenter trauma data. An early example was the establishment of the Vietnam Vascular Registry, with subsequent landmark reports on the epidemiology and outcomes of a wide variety of vascular injuries.5 The recognized importance of the prospective collection of data from wartime experiences led to the establishment of the Joint Theater Trauma System and the Joint Theater Trauma Registry, which continues to collect critical information from combat operations in Iraq and Afghanistan. The past decade has also seen the establishment of the National Trauma Data Bank (NTDB) by the American College of Surgeons. The NTDB is the largest trauma registry ever assembled, and as of the 2012 annual report, it contains more than 5 million records from 744 trauma centers in North America. This rich data source has allowed detailed descriptive data and adequately powered statistical analysis of a wide variety of vascular injuries and related traumatic disease.
Trauma has become the leading cause of death worldwide and represents the leading cause of death among young adults in industrialized nations. In the United States in 2010, trauma was the cause of death in 63% of patients aged 1 to 24 years and 42% of the 25- to 44-year age group. Trauma results in more losses of productive life years than any other disease process, and an analysis of the economic impact of trauma estimated yearly costs of $406 billion. The overall incidence of vascular injury from trauma in recent NTDB analyses is 1.6% for adults and 0.6% for pediatric patients, which is significantly lower than the 6% to 12% incidence among combat casualties.6,8 The majority (60%-90%) of both truncal and extremity civilian vascular injuries are due to penetrating mechanisms, most commonly handguns or other firearms. Vascular injury with blunt trauma is relatively uncommon and is typically associated with higher force or velocity trauma, advanced age, extremity soft tissue or bone injuries, and intra-abdominal or thoracic organ injury. Current military data indicate that most vascular injuries are due to blast or explosive events (73%) and frequently have a combined injury to the associated vein (58%). Although the main focus of this chapter is traumatic injury, several series have identified major increases in iatrogenic trauma from percutaneous endovascular procedures or laparoscopic procedures, accounting for up to half of all vessel injuries in some centers.
Major vascular disruption or bleeding continues to be associated with approximately 25% of early trauma deaths, and some important modern epidemiologic trends have been identified. The average age of all trauma patients and those with vascular injury is steadily increasing, with a 10-year increase in the average age of trauma patients between 1996 and 2004.9 Thus, the classically described “young and healthy” trauma patient is being replaced with more elderly patients who have a higher incidence of preexisting vascular disease that may increase the risk for vessel injury and alter treatment options. Decreased transport times and improved prehospital care are resulting in more severely injured patients with major vascular injury reaching a hospital alive, and the ratio of prehospital to in-hospital deaths has decreased from 1.5 to less than 0.5.9 For severely injured patients, damage control techniques including abbreviated surgery, hemostatic resuscitation, and temporary intravascular shunts (both arterial and venous) have been associated with major reductions in both mortality and limb salvage.10,11 Arguably the most significant development in modern vascular surgery is the emergence of endovascular techniques for managing vascular disease, and these techniques are now being extended to traumatic injuries. Although it was initially limited to injuries in which open repair is highly morbid (thoracic aorta) or provides limited exposure (distal carotid), endovascular control of hemorrhage with definitive repair is now an option for almost all arterial injuries. A national analysis demonstrated a 27-fold increase in the use of endovascular therapy, and this was associated with a decrease in morbidity, hospital stay, and mortality.12 Endovascular techniques have even now been extended to the combat setting and include vena cava filter placement, embolization (58% of nonfilter treatment procedures), and stent placement (30%). Technical success of these procedures is 90% to 100% and provides a definitive, minimally invasive treatment option.13
Mechanism of Injury, Ballistics, and Biomechanics
The clinical presentation, pattern of associated injuries, need for intervention, and outcomes after traumatic vascular injury will be highly dependent on the mechanism of injury and the specific characteristics of that mechanism. For blunt injury, this mainly involves the velocity or forces of impact, the use of restraints or protective devices, and the primary anatomic areas that sustain the brunt of the kinetic forces. In addition, patients sustaining blunt trauma may suffer penetrating-type vascular injury from impalements, cuts from glass or sharp debris, and puncture by fractured bone fragments. The most common blunt mechanism associated with major vascular injury is motor vehicle collision, and injury to the vessel typically results from stretching and shearing forces or from vascular avulsion (usually due to rapid deceleration).14 Several additional etiologic factors have been proposed, including the generation of a sudden spike in intravascular pressure, creating wall stress and tear or rupture, and forward inertial deceleration of blood impacting the anterior vessel wall (“water-hammer” effect).14,15
For penetrating trauma, the wounds can be primarily classified as due to stab/puncture or from missiles/projectiles that are typically firearms related. Stab and puncture wounds result in direct vascular injury without significant transmission of kinetic energy or damage to surrounding tissues. Missiles may injure vascular structures by direct laceration or by transfer of energy due to proximity, with an impact kinetic energy (iKE) related to the mass (M) and velocity (V) of the projectile (iKE = 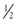 MV2). Rarely, missiles or fragments can enter the lumen of the arterial or venous system and embolize, resulting in luminal occlusion and distal ischemia. Terms such as “high velocity” are often used but are frequently poorly defined or misunderstood, and there are multiple projectile characteristics, such as shape, deformity, fragmentation, pitch, and yaw, that are of equal importance to or more important than the velocity.16 Additional injury to surrounding tissue has been attributed to both the “sonic wave” and the stretching of tissue due to the pressure wave (“temporary cavity”), considerations that have been used to justify excessive tissue débridement or vessel resection. However, subsequent study has found that the effect of the sonic wave is negligible and the size of the temporary cavity is smaller (11× diameter of the missile) than previously described (up to 30× diameter).16 Jahnke et al17 examined the arteries from vascular wounds in the Korean War and excised the injured segment as well as a 1-cm margin of grossly normal vessel. They demonstrated histologic evidence of injury that extended through the 1 cm of normal-appearing vessel. Similarly, histologic vessel injury was demonstrated in samples from the Vietnam War but showed no correlation with the outcome of repair. Excessive débridement of grossly normal vessel or surrounding tissues is not required, even with the ballistics of so-called high-velocity wounds. Finally, particular mention should be made of shotgun injuries. Although shotguns are classified as low velocity, the resultant tissue injury due to diffuse tearing, contusion, and devascularization from multiple pellets can be significantly greater than even that of high-velocity gunshots.18,19 Although less common than stab or gunshot mechanisms, they have been associated with vascular injury in up to 25% of wounds, and the degree of injury is highly correlated with the type of pellet and the effective weapon-victim range.18 Shotgun pellets are notorious for creating vessel micropunctures that are difficult to diagnose even by examination or imaging studies.20
MV2). Rarely, missiles or fragments can enter the lumen of the arterial or venous system and embolize, resulting in luminal occlusion and distal ischemia. Terms such as “high velocity” are often used but are frequently poorly defined or misunderstood, and there are multiple projectile characteristics, such as shape, deformity, fragmentation, pitch, and yaw, that are of equal importance to or more important than the velocity.16 Additional injury to surrounding tissue has been attributed to both the “sonic wave” and the stretching of tissue due to the pressure wave (“temporary cavity”), considerations that have been used to justify excessive tissue débridement or vessel resection. However, subsequent study has found that the effect of the sonic wave is negligible and the size of the temporary cavity is smaller (11× diameter of the missile) than previously described (up to 30× diameter).16 Jahnke et al17 examined the arteries from vascular wounds in the Korean War and excised the injured segment as well as a 1-cm margin of grossly normal vessel. They demonstrated histologic evidence of injury that extended through the 1 cm of normal-appearing vessel. Similarly, histologic vessel injury was demonstrated in samples from the Vietnam War but showed no correlation with the outcome of repair. Excessive débridement of grossly normal vessel or surrounding tissues is not required, even with the ballistics of so-called high-velocity wounds. Finally, particular mention should be made of shotgun injuries. Although shotguns are classified as low velocity, the resultant tissue injury due to diffuse tearing, contusion, and devascularization from multiple pellets can be significantly greater than even that of high-velocity gunshots.18,19 Although less common than stab or gunshot mechanisms, they have been associated with vascular injury in up to 25% of wounds, and the degree of injury is highly correlated with the type of pellet and the effective weapon-victim range.18 Shotgun pellets are notorious for creating vessel micropunctures that are difficult to diagnose even by examination or imaging studies.20
Blast injury, such as that seen with improvised explosive devices in the wars in Iraq and Afghanistan, has its own unique wounding pattern and mechanisms. The overall mechanism is a combination of blunt force trauma, penetrating fragment injuries, and potentially thermal injury. Blast injuries in modern combat series now outnumber standard gunshot wounds and account for 73% of vascular injuries among wounded soldiers. Blast effects are classified as primary (direct blast pressure), secondary (penetrating fragments), tertiary (collision with objects or vehicles), and quaternary (thermal injury). The relative distribution of each varies by type of explosive, enclosed versus outdoors, and presence of protective equipment. The majority of vascular and other injuries are due to secondary and tertiary blast effect (81%). Blast-induced extremity vascular injury with coexisting fracture is associated with a 50% amputation rate with attempts at limb salvage and a 77% amputation rate overall.21 Blast injuries may also occur with civilian incidents such as terrorist bombings, and vascular injuries have been identified in up to 10% of victims.
Vascular Injury Classification and Scoring
The ideal vascular injury classification and scoring system has yet to be developed, probably because of the wide variation in traumatic vascular disease and associated outcomes. Standard injury scoring systems such as the Injury Severity Score and the Revised Trauma Score have been shown to significantly underestimate mortality among patients with major vascular injury.
For battlefield or combat-related vascular trauma, it is probably most useful to categorize injuries according to a three-tiered system based on the wound location and ability to achieve hemorrhage control. This system can also easily be applied to civilian vascular trauma. Peripheral or extremity wounds (tier 1) are located distal to the axillary artery or vein (upper) or common femoral artery or vein (lower) and are responsible for the smallest percentage (14%) of modern battlefield deaths due to bleeding.22 This has largely been attributed to the widespread distribution and use of tourniquets or pressure packing, performed in 56% of combat casualties. Proximal groin or axillary wounds (tier 2) are now categorized as junctional wounds and are often not amenable to either direct pressure or tourniquets. This has led to the development and wide deployment of advanced hemostatic dressings and the search for junctional hemorrhage control devices.22 The third category (tier 3) is intracavitary wounds to the thorax or abdomen and pelvis, resulting in noncompressible truncal hemorrhage (NCTH). This has been identified as the most difficult and deadly source of hemorrhage on the battlefield, and NCTH has been identified as the etiology in 67% of battlefield deaths from hemorrhage.22 This categorization scheme is most useful for penetrating or battlefield trauma, but it can be applied to any vascular trauma with associated hemorrhage.
The American Association for the Surgery of Trauma Organ Injury Scaling (AAST-OIS) system is the most widely used grading system for traumatic injuries and is well validated for predicting outcomes and need for intervention in solid organ injuries. AAST-OIS tables are currently also available for traumatic vascular injuries, including cervical, thoracic, abdominal, and peripheral. This grading system is organized primarily around the exact identity of the vessel rather than the severity of the vascular injury or the degree of hemorrhage or ischemia and thus provides little additional information about treatment or outcomes. Alternative schemes, such as the grading system developed by Biffl et al23 for blunt carotid injuries, may be more useful for guiding treatment and predicting outcome but will probably need to be specifically developed for individual vessels and even mechanisms.
For major extremity fractures and soft tissue injury, several scoring systems have been developed to characterize the “mangled extremity.” The most commonly described include the Mangled Extremity Severity Score, the Mangled Extremity Syndrome Index, the Predictive Salvage Index, and the Limb Salvage Index.24 Although they contain different components, the presence of vascular injury and limb ischemia is a universal key variable. In addition to providing an objective classification system for epidemiologic purposes, these scores have been studied for their ability to predict the need for extremity amputation in both civilian and military settings.24,25 Although they have been correlated with the need for amputation, prospective trials have found that they lack adequate predictive ability to be used for individual patients and did not correlate with limb salvage when arterial reconstruction was performed.25
Epidemiology, Clinical Presentation, and Diagnosis of Vascular Injury
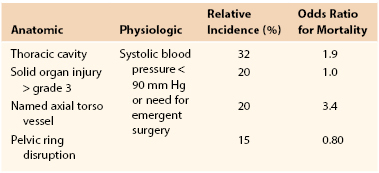
The clinical presentation of traumatic vascular injury is highly variable and depends on the type of injury, anatomic area or body cavity, associated injuries, structures or organs being perfused, presence of alternative or collateral blood supply, and presence of intoxication or altered mental status. Diagnosis depends on an orderly and prioritized evaluation including a focused history, physical examination, and selective use of imaging modalities. Ongoing or life-threatening hemorrhage is assessed during the primary survey, and assessment for any other manifestations of vascular injury is performed in the secondary survey and imaging. Vascular injuries are frequently associated with trauma to local musculoskeletal or other anatomic structures or organs, and thus recognition of these typical injury patterns or clusters is critical to early diagnosis and avoiding missed injuries. Table 155-2 provides a listing of these common associations for select named vessels. Diagnostic or “hard” signs of vascular injury include arterial bleeding, a pulsatile hematoma, absence of pulses or limb ischemia, and a bruit or thrill indicative of arteriovenous fistula. Suggestive or “soft” signs of vascular injury include a nonpulsatile hematoma, decreased pulses or pressure index, unexplained anemia or hypotension, and injury to closely associated structures (typically nerve) or close to the vessel. Vascular injuries can also be present in the absence of any hard or soft signs, and these “occult” vascular injuries are being increasingly recognized with routine imaging but are of unclear clinical significance.
Table 155-2
Patterns of Associated Musculoskeletal and Other Structures with Specific Vessel Injury
| Vessel | Musculoskeletal | Other |
| Carotid artery | Cervical spine Mandible, Le Fort II/III facial fracture Skull base | Vertebral vein Carotid artery Trachea, esophagus |
| Vertebral artery | Cervical spine (vertebral foramina) Skull base | Jugular vein Carotid artery |
| Subclavian artery or vein | Clavicle Sternum, manubrium | Thoracic duct (left) Brachial plexus, recurrent laryngeal nerve |
| Axillary artery or vein | Shoulder, proximal humerus | Brachial plexus, axillary nerve |
| Brachial artery | Midhumerus Biceps, triceps | Ulnar nerve Median nerve |
| Radial or ulnar artery | Elbow fracture or dislocation Radius, ulna, wrist Forearm and hand flexor tendons | Distal radial nerve (sensation only) Ulnar nerve |
| Thoracic great vessels | Sternum, manubrium | Innominate vein, recurrent laryngeal nerve |
| Descending aorta | Thoracic spine Posterior rib fracture or dislocation Diaphragm | Esophagus Lung Left subclavian vein (blunt) |
| Abdominal aorta or vena cava Suprarenal Infrarenal | Thoracic or lumbar spine T12-L2 (with or without spinal cord injury) L2-sacral fractures | Zone 1 retroperitoneal hematoma Stomach, transverse colon, pancreas Duodenum, small bowel |
| Portal vein or superior mesenteric vein | Lumbar spine fracture or ligament injury Rib fractures | Zone 4 retroperitoneal hematoma Duodenum (second or third portion), head of pancreas Portal triad (hepatic artery, common bile duct) |
| Renal artery or vein | Lumbar spine Posterior rib fracture or dislocation | Zone 2 retroperitoneal hematoma Kidney, proximal ureter, adrenal or gonadal vessels |
| Iliac vessels | Pelvic fracture Sacral fracture Sacroiliac joint disruption | Zone 3 retroperitoneal hematoma Cecum (right), sigmoid colon (left) Bladder, ureters |
| Femoral artery or vein | Pelvic fracture Acetabulum Proximal to mid-femur | Femoral nerve, sciatic nerve (rare) Inguinal ligament Spermatic cord |
| Popliteal artery or vein | Dislocated or “floating” knee Distal femur, proximal tibia | Tibial nerve Calf compartment syndrome |
| Tibioperoneal vessels | Tibia, fibula Ankle fracture or dislocation | Tibial nerve, peroneal nerve (footdrop) Calf compartment syndrome |
Hard signs of vascular injury on physical examination are highly specific, particularly with penetrating extremity trauma (100%).26,27 Similarly, hard signs of arterial injury have been validated as nearly 100% specific in penetrating neck trauma but are present in only 20% of patients.27 The human body has a remarkable system for controlling hemorrhage that is mainly mediated by vascular tone and clot formation. A completely severed artery will often retract into surrounding tissue and constrict, with resultant clot formation and hemostasis. Alternatively, partial lacerations have a higher propensity for continued bleeding. The hard signs of injury will obviously be less applicable in intracavitary thoracic or abdominal vascular injury, with hypotension being the primary indicator of active bleeding. Initial hypotension in the emergency department or operating room is present in 25% of abdominal or thoracic vascular trauma, and if it is present, it is associated with an 86% mortality.28 Overall, less than 10% of vascular injuries will be manifested with hard signs, and the majority will have either soft signs or delayed presentation or be asymptomatic.26,27 Although soft signs of injury have been associated with up to a 63% incidence of vascular injury, the reliability of individual soft signs varies widely.18 The most commonly identified soft sign of vascular injury is a proximity wound, which historically mandated angiography or surgical exploration. Although proximity wounds have up to a 10% incidence of associated vascular injury, the injuries typically either are insignificant or have accompanying findings on physical examination.18
The reported reliability of physical examination for identifying significant peripheral vascular lesions in civilian trauma is excellent and can be used to select patients for operation, additional imaging, or observation only.26,29,30 However, recent data from the military indicate that the physical examination alone may be much less reliable for combat injuries, with a reported sensitivity of only 38% and negative predictive value of 51%.31 The physical examination is also less reliable in identifying vascular injury from shotgun wounds, and routine imaging is recommended on the basis of proximity even with normal examination findings. The reliability of physical examination is also highly dependent on whether minor and typically clinically insignificant vascular lesions are included. Simple bedside measurement of the injured extremity pressure indexed to a normal limb, including ankle-brachial or wrist-brachial index, has been prospectively validated as 100% sensitive for identifying penetrating extremity vascular injury.32 Smaller series have confirmed the utility of the ankle-brachial index in blunt extremity trauma, and a value of less than 1 is suggestive of injury and should prompt further evaluation or imaging studies.29 Overall, the combination of physical examination and pulse index measurement (for extremity wounds) appears to reliably identify or to exclude the majority of vascular injuries that will require some type of intervention or become symptomatic.32
Head, Face, and Neck
Intracranial injury is typically a combination of injury to the dural arterioles or veins and brain parenchyma and is beyond the scope of this chapter. Vascular injuries of the face are due either to direct penetrating injury to branches of the external carotid artery or to blunt trauma associated with major facial fractures. The clinical presentation is usually obvious, with either external or intraoral/nasal hemorrhage, and commonly associated with aspiration or airway compromise.
The incidence of vascular injury in the neck is 20% with penetrating trauma and approximately 1% in blunt trauma.23,33 The clinical presentation of cervical vascular injury demonstrates a marked polarity between penetrating and blunt mechanisms. Multiple studies have validated that physical examination is reliable in identifying major cervical vascular injuries with penetrating trauma. Frykberg et al26 reported a positive predictive value of 90% for hard signs, and overall the physical examination had a low missed injury rate of 0.7%, which is comparable to results with routine arteriography. The exact opposite is true of blunt cerebrovascular injuries, with a majority of patients demonstrating no hard signs of hemorrhage and presenting with neurologic deficits (28%), developing deficits after a significant delay (up to 78%), or remaining entirely asymptomatic (up to 40%).23,33 There has been significant debate about the optimal modality for diagnosis of blunt cervical vascular injury, and until recently standard angiography was thought to be optimal.23 Multiple series have now confirmed the accuracy and reliability of computed tomography (CT) angiography for cervical vascular injury, and it is widely considered the screening study of choice.34,35 Similarly, the routine use of angiography for penetrating neck injury, particularly for zone 1 and zone 3 injury, has been largely replaced by CT angiography.
Torso
Noncompressible Truncal Hemorrhage
Active bleeding from abdominal or thoracic structures carries a high associated mortality, accounting for 60% to 70% of potentially preventable mortality in the civilian setting and 80% in the combat setting.36,37 A recently described set of criteria for NCTH has been developed and is outlined in Table 155-3.37 By use of these definitions, 13% of battlefield casualties met anatomic criteria and 2% were identified as having NCTH. The most lethal of these patterns was injury to a named torso vessel (odds ratio, 3.4), which was present in 20% of NCTH cases. A similar incidence of NCTH of around 2% has been demonstrated in civilian trauma but with a higher prevalence of blunt traumatic mechanisms. Similar to combat statistics, NCTH accounts for a large percentage (45%-85%) of preventable deaths from bleeding. Unlike peripheral or junctional vascular trauma, these injuries are not amenable to direct compression or simple inflow occlusion (tourniquet) in the prehospital or emergency department phase and thus have been a focus of great interest and innovation. Critical concepts or interventions for patients with NCTH are (1) minimizing delays in transfer from the emergency department to the operating room (or directly to operating room resuscitation), (2) permissive hypotension until vascular control is obtained, (3) balanced or “hemostatic” resuscitation with early use of plasma, (4) procoagulant adjuncts such as tranexamic acid, and (5) use of damage control surgery techniques and intravascular shunts when indicated.10,38 Innovative work that could have a direct impact on survival from NCTH is being done and includes the use of endovascular techniques such as aortic balloon occlusion for temporary vascular control and the development of pharmacologic metabolic or hormonal agents to induce a “pro-survival” phenotype that is more tolerant to severe hemorrhage.
Thoracic
Injuries to named thoracic vessels from either blunt or penetrating mechanisms are highly morbid, with a large percentage of patients dying before they reach a hospital. A study of 1203 battlefield casualties in Iraq and Afghanistan found no survivors among those with injury to a named abdominal or thoracic vessel.39 Even for pediatric patients, who are typically more tolerant to injury and hemorrhage, mortality approaches 100% with thoracic vascular injuries and hemodynamic instability at presentation. The majority of thoracic great vessel injury (90%) is due to penetrating trauma but is often rapidly fatal, and 28% of penetrating aortic injuries are in the thoracic portion.40 In a landmark study of 5760 vascular injuries, Mattox et al41 found that 20% involved the thoracic vessels, most commonly penetrating (89%) wounds of the arteries (70%). A bullet or missile that traverses the intrapleural thorax may either graze or penetrate a thoracic artery or vein, causing either a contained hematoma or active bleeding with continuing blood loss. Knife or spear-type wounds depend on the angle at which the weapon enters the thorax, the depth to which it penetrates, and the body habitus of the patient. Impalement-type injuries surprisingly cause little vascular damage as the impaling object pushes many of the structures aside as it penetrates. In the modern era of high-velocity vehicular trauma, the single most common thoracic vascular injury seen among survivors to hospital admission is blunt injury to the thoracic aorta.42 A testament to the importance of high velocities and impact forces is that blunt aortic injury is identified in up to 30% of airplane crash victims.43 Diagnosis is largely dependent on the type of injury, and a chest radiograph may identify signs of mediastinal or pleural hemorrhage but cannot definitively rule out an injury.44
CT angiography has now largely replaced standard angiography as the study of choice among hemodynamically stable patients.42,45 Hard signs of vascular injury or active bleeding should prompt immediate operation, and transporting the patient directly to the operating room or a hybrid operating room–endovascular suite may improve survival.38,46 The use of advanced endovascular techniques in vascular trauma has generally lagged behind the elective applications. However, endovascular intervention with stent-graft repair has rapidly emerged as the preferred treatment modality for a large percentage of thoracic vascular injuries, particularly blunt injury to the thoracic aorta.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree