Chapter 43
Local Complications
Aortoenteric Fistula
Ross Milner, Samantha Minc
Based on a chapter in the seventh edition by Vikram S. Kashyap and Patrick J. O’Hara
Aortoenteric fistula (AEF) is defined as a communication between the aorta and gastrointestinal (GI) tract. AEF is classified as primary or secondary on the basis of the underlying cause leading to the fistula development. Primary AEF is a communication between the native aorta and GI tract; secondary AEF is a communication between a reconstructed aorta (for either aneurysmal or occlusive disease) and the GI tract.
Sir Astley Cooper was the first to describe a primary AEF in a publication in 1829. He referred to it as a “sometimes but serious complication of an aneurysmal aorta.”1 Brock published the first report of a secondary AEF in 1953, wherein he described a fistula between the proximal anastomosis of an aortic homograft and the duodenum.2 Zenker performed the first repair of a primary AEF in 1954 by primary closure,3 and MacKenzie et al4 performed the first successful repair of a secondary AEF in 1958.
AEF presents a particularly complicated challenge for the vascular surgeon. When it is left untreated, the outcome is almost universally fatal. However, surgical repair is fraught with complications, and despite continued advances in medicine and critical care, morbidity and mortality rates remain high.
In addition to the difficulties encountered with surgical treatment, AEF is notoriously difficult to diagnose, and as such, a high index of suspicion is required in approaching any patient with GI hemorrhage and history of aortic disease. This chapter reviews the pathogenesis, etiology, diagnostic methods, and treatment options for AEF.
Primary Aortoenteric Fistula
Incidence, Etiology, and Pathogenesis
The incidence of primary AEF has been reported at 0.04% to 0.07% in large autopsy series.5 The body of literature available on the subject of primary AEF is small, with approximately 251 cases available in the published literature.
Despite the rarity of primary AEF in the population overall, the incidence in patients with aneurysms of the abdominal aorta is 0.69% to 2.36%.6 In the majority of cases (83%), an aneurysmal aorta is associated with primary AEF; foreign bodies, tumors, radiotherapy, infection (historically due to tuberculosis and syphilis but now most commonly caused by Klebsiella and Salmonella), and GI tract disease (peptic ulcer disease and perforating biliary stones) account for the remainder of AEF. The mean diameter of the aorta with primary AEF is 6.2 cm; the mean age of patients is 64 years, with a male-to-female ratio of 3 : 1.7
The most commonly described GI tract location for primary AEF is the third and fourth portion of the duodenum (54%). It is presumed that this is due to the tethering effect of the ligament of Treitz, leaving this portion of the duodenum exposed to the direct pulsatile pressure of the aorta. Primary AEF has also been described in the following locations: esophagus (28%), small and large bowel (15%), and stomach (2%).7
Overall, the pathogenesis of primary AEF is uncertain. The proposed mechanisms are mechanical, infectious, and inflammatory (Fig. 43-1). In the majority of cases, the mechanical component is caused by the pulsatile pressure of an expanding aorta against the wall of the GI tract. This leads to local compression and ischemia, with weakening of the wall and eventual erosion with fistula formation.
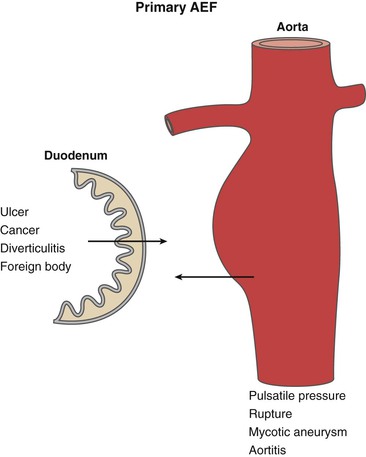
Figure 43-1 Etiologic factors for primary aortoenteric fistula (AEF). Pulsatile pressure from an expanding aneurysm is thought to be the predominant feature in the development of primary AEF. Other factors related to intrinsic bowel conditions may play a role in the development of primary AEF in a minority of cases.
Secondary Aortoenteric Fistula
Incidence and Etiology
Secondary AEF is more common than primary AEF and is related to prior vascular surgery, with an incidence of 0.36%8 to 1.6%9 reported after open abdominal aortic graft reconstruction. The interval from aortic reconstruction to onset of symptoms is on average 2 to 6 years after graft placement. Secondary AEF involves fistulization between the GI tract and a prior vascular reconstruction for either aneurysmal or occlusive disease. Although it is typically described as occurring in the setting of a synthetic graft, secondary AEF has also been reported with aortic homograft reconstruction and allografts as well. Similar to primary AEF, the most common location described for secondary AEF is the distal duodenum and proximal jejunum. However, secondary AEF has been described at multiple GI sites, depending on the location of the prosthetic graft.
Secondary AEF has been further classified on the basis of the location of the fistula with regard to the suture line of the graft (Fig. 43-2). A fistula that has a direct communication between the arterial circulation and the GI tract at the level of the suture line is classified as a graft enteric fistula. Communication between the GI tract and the graft interstices (but not at the suture line) is referred to as a graft enteric erosion. Both entities are highly morbid complications that require urgent management; however, the etiology and presentation may differ. The graft enteric fistula’s involvement of the suture line will disrupt the arterial anastomosis and cause dramatic hemorrhage, whereas graft enteric erosion may be manifested first with infectious symptoms as a result of direct contact of the graft material with the GI tract, allowing bacterial translocation to occur between the interstices of the graft from the GI tract.
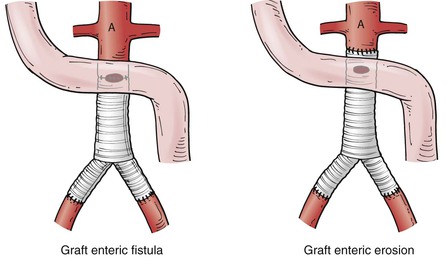
Figure 43-2 Graft enteric fistula versus graft enteric erosion. Graft enteric fistula has a communication between the bowel and the arterial circulation at the level of an arterial prosthetic suture line. Graft enteric erosion has a communication between the bowel and the interstices of an arterial prosthetic graft without involvement of the suture line.36
The etiology of secondary AEF includes both patient and surgical factors that occur during the initial placement of the graft. A history of multiple vascular procedures, wound complications, infection, emergency operation, and technical error have been found to predispose to secondary AEF formation.10
Pathogenesis
The proposed mechanisms of secondary AEF pathogenesis are infection, pulsatile pressure, and technical error.
Infection
Infection, introduced either at the time of initial surgery or through bacteremia, is an important cause of secondary AEF formation. Graft infection leads to local inflammation and results in breakdown of the suture line, pseudoaneurysm formation, and eventual rupture into surrounding structures. The majority of patients described in case reports with AEF have clinical and bacteriologic evidence of graft infection at the time of reoperation. A study by Busuttil et al11 demonstrated the role of infection in the pathogenesis of AEF with a canine model of 24 animals divided into four groups. In group 1, the duodenum was fixed to the proximal suture line of a Dacron aortic interposition graft. Group 2 underwent the same procedure but was subjected to an infusion of Staphylococcus aureus. In group 3, a false aneurysm was created with a patch of duodenum incorporated onto the anterior aspect of the proximal suture line. The same procedure was performed in group 4; however, this group was also subjected to S. aureus infusion. At the end of 6 weeks, no dogs in group 1 had an AEF, two of six dogs in group 2 had an AEF, three of six dogs in group 3 had an AEF, and five of six dogs in group 4 had an AEF. This study clearly demonstrated that infection is a key factor in the formation of AEF, independent of any mechanical or technical factors.
Pulsatile Pressure
Pulsatile pressure also remains a factor in the proposed mechanism of AEF formation. As described earlier, pressure from a noncompliant prosthesis against the bowel wall may lead to ischemia to the surrounding tissue and eventual erosion. Another mechanism described is suture line disruption, leading to the formation of an expanding pseudoaneurysm, compressing surrounding structures and eventually eroding into the bowel. Despite the intuitive nature of this mechanism, the majority of studies continue to point to the issue of infection as the root cause of AEF.
Technical Error
The final factor in the formation of AEF is technical error. This includes inoculation of the prosthesis at time of implantation as well as duodenal injury, serosal thinning, and ischemia during the operation. Changes in standards in patient preparation and appropriate antibiotic administration intraoperatively have decreased the overall rate of aortic graft infection. Operative trauma to the bowel, however, remains an issue. The bowel can be injured during the initial dissection and exposure, either directly with sharp injury and heat transfer from cautery or indirectly by overretraction and tension. Retraction injury may occur from the retractor blades directly; however, it may also occur by overcompression of the bowel, causing ischemia, or by allowing the bowel to become dehydrated outside of the body. Methods to reduce bowel trauma include wrapping the bowel in warm moist laparotomy sponges during the procedure (being mindful to resoak the sponges intermittently), keeping the bowel intraperitoneal by packing it above the liver (which can be difficult in larger patients), and using an isolation bag to keep the bowel moist and protected (but still visible) while it is outside the abdomen. Another method to reduce bowel trauma is by using a retroperitoneal approach and interposing tissue between the aorta and the graft at the end. Proponents of the retroperitoneal approach believe that avoiding division of the parietal peritoneum to expose the aorta limits the devitalization of tissue and development of adhesions between the graft material and bowel. With regard to interposition of tissue between the aorta and graft, aortic sac closure followed by a second layer of retroperitoneal tissue and parietal peritoneum is the preferred approach. If that is unavailable, a flap of greater omentum or a layer of bovine pericardium patch material may be used. Omental coverage may be necessary more commonly in end-to-side graft anastomoses to the aorta, rather than in end-to-end anastomoses, and in thin patients.
Aortoenteric Fistula after Endovascular Repair
There is a growing body of literature documenting reports of AEF after endovascular aneurysm repair (EVAR) and thoracic endovascular aneurysm repair (TEVAR). There have been approximately 20 aortoenteric fistulae reported in the case of EVAR and 20 aortoesophageal and aortobronchial fistulae reported in the case of TEVAR. Proposed mechanisms in the case of endovascular repair include persistent endoleak with growth of the residual sac, multiple coiling efforts to repair an endoleak, erosion of the stent-graft through the aorta, endotension, and infection at the time of graft placement.12,13
Clinical Presentation
The classic clinical triad for primary AEF as described by Sir Astley Cooper in 1829 consists of GI bleeding, abdominal pain, and a pulsatile mass. Recent reviews note the incidence to be 64% to 94% for GI bleeding, 32% to 48% for abdominal pain, and 17% to 25% for a pulsatile abdominal mass, with all three of these symptoms occurring concurrently in only 11% of cases.14 Other symptoms reported include back pain, fever, and sepsis. Laboratory findings have been reported in only half of the studies, and only two thirds of patients demonstrated a hemoglobin level lower than normal (8 mmol/L). One quarter of patients had leukocyte counts above the upper limit of normal (10 × 109).11
In the case of secondary AEF, hemorrhage remains an important feature of the presenting symptoms. In a recent retrospective review of 4137 patients who underwent aortic reconstruction between 2000 and 2008, 37 patients (0.08%) presented with secondary AEF. Of these patients, 73% presented with some form of hemorrhage, 41% with sepsis, 22% with abdominal or back pain, 14% with graft limb thrombosis, 11% with a groin fistula, 8% with a peripheral abscess, 5% with a femoral pseudoaneurysm, and 5% with peritonitis.15
In both primary and secondary AEF, a classic clinical feature is the “herald bleed.” This is a minor bleed that is self-limited because of vasospasm and thrombus formation. Herald bleeds may lead to hospital admission immediately; however, some patients experience multiple episodes of recurrent bleeding. Regardless of the initial presentation, an untreated herald bleed will commonly be followed by an exsanguinating bleed within hours to months.
Diagnosis
The diagnostic approach to the evaluation of AEF is dependent on the patient’s hemodynamic status on presentation. In general, a high level of suspicion should be maintained in approaching any patient with massive GI bleeding and a history of an aortic aneurysm or previous aortic revascularization. In such cases of massive bleeding, diagnosis will often be made during exploratory laparotomy. If the patient is stable, the three major diagnostic modalities are computed tomography (CT) with iodinated contrast enhancement, esophagogastroduodenoscopy (EGD), and angiography. There has been some controversy in the past about which study should be done first; some authors have recommended EGD, followed by CT scan, followed by visceral angiography if no source is found. However, with the improvements of radiologic imaging, CT scan has become a preferred initial diagnostic test; CT scans are less invasive than EGD or angiography, are easy to obtain, and do not risk thrombus dislodgement.16
Investigations
Computed Tomography
Common findings on CT scan include effacement of the fat planes around the aorta, perigraft fluid and soft tissue thickening, ectopic gas (Fig. 43-3), tethering of adjacent thickened bowel loops toward the aortic graft, and, in rare cases, extravasation of contrast material from the aorta into the involved segment of bowel.17 The detection rate of CT for AEF is the highest of all modalities (61%),12 and CT also can be used to assess the peripheral vasculature to assist with revascularization plans during repair.
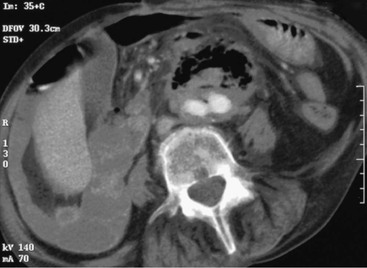
Figure 43-3 Contrast-enhanced CT scan of a patient with a secondary aortoenteric fistula. Note the accumulation of gas and retroperitoneal inflammation surrounding a bifurcated aortic prosthesis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


