Vascular Pharmacology PGs are eicosanoid-compounds synthesised from arachadonic acid (normally found bound to cell membrane phospholipids). After injury, arachadonic acid (AA) is liberated from the cell membrane by the enzymatic action of phospholipase A2 [PA2]). Once liberated, free-AA may enter the cyclooxygenase (COX) pathway whereby COX enzymes transform AA into various PG’s. There are two broad categories of active COX: COX-1 and COX-2. COX-1 is constitutively expressed in most tissues including gastrointestinal tract (GIT), platelets and kidney. COX-2 is mostly an inducible enzyme in response to injury (including endothelial injury) and inflammatory stimuli, and a major source of prostanoids. PGs have numerous effects, including acting as inflammatory mediators. Numerous PGs are also active in vascular tissue contributing to vasodilatation (PGI2, PGE2), vasoconstriction (PGF2α, thromboxane A2 [TxA2]), platelet aggregation (TxA2) and platelet inhibition (PGI2).
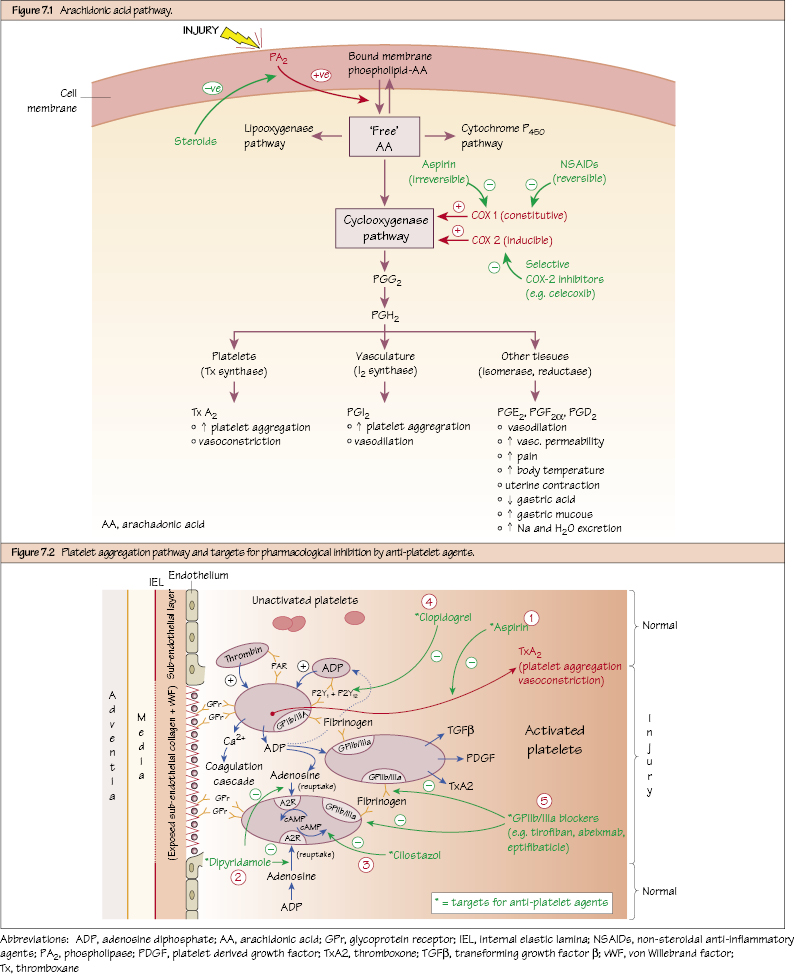
Arachidonic Acid Pathway
Targeting the Arachadonic Acid Pathway
Platelet Aggregation
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


