Chapter 25
Vascular PET CT and SPECT CT
Simona Ben-Haim, Ora Israel
Based on a chapter in the seventh edition by Kristofer M. Charlton-Ouw and Alan B. Lumsden
Traditional imaging modalities for the assessment of blood vessels have focused on size, irregularity of the vascular lumen, and anatomic changes in adjacent structures. The ability of these morphologic imaging techniques to identify physiologic changes such as active inflammatory processes and plaques at risk for rupture is, however, limited. Molecular imaging has the advantage of enabling noninvasive physiologic assessment of these processes with radionuclides, very often early in the course of the disease. Such early assessment can lead to subsequent changes in the clinical management, potentially affecting patients’ outcomes. The limitations of traditional nuclear medicine techniques, mainly low spatial resolution and lack of anatomic details, have been overcome in the last decade with the introduction of the hybrid single-photon emission computed tomography/computed tomography (SPECT/CT) and positron emission tomography (PET)/CT imaging devices. A large body of evidence has accumulated on the use of molecular probes for the assessment of vascular inflammation and infection, for early diagnosis, for precise determination of the extent of disease, and for assessment of response to therapy.
Basic Principles
Molecular Imaging of Atherosclerotic Plaques
Molecular imaging of atherosclerosis, including SPECT and PET techniques, takes advantage of the wide array of biologic mechanisms involved in the different stages of atherosclerosis and plaque formation. Many of the molecular probes developed for plaque imaging are linked with inflammation, a major component of vulnerable plaques.1
Challenges
There are challenges in molecular imaging of vascular plaques. The small size of such plaques (most are less than 5 mm in diameter) is close to the resolution limit of SPECT and PET. Also, some arteries are located deep within the body, and in some regions, such as the coronary arteries, imaging is of lower quality because of artifacts related to cardiac and respiratory motion.2 Various radiotracers have been used for the assessment of atherosclerosis. Most of the radiopharmaceuticals labeled with single-photon emitters have unfavorable imaging properties, including slow blood clearance and low ratio of target (atherosclerotic plaque) to background (blood pool), or demonstrate affinity with nonspecific binding sites. Despite the large variety of probes labeled with single-photon emitters, none is being widely used in the clinical setting to identify vulnerable patients. More research is needed to identify a suitable probe that will also yield a robust clinical imaging tool. Advances are being made, however, as described here.
LDL-Macrophage Labeling
Low-density lipoprotein (LDL) accumulates in the lipid core of atherosclerotic plaques.3 TechnetiumTc99m (Tc99m)–labeled LDL has been shown to accumulate in experimental atherosclerotic plaques in rabbits.4 In clinical studies a fourfold greater uptake was found in carotid endarterectomy macrophage-rich specimens than in fibrotic specimens.5 Owing to slow blood clearance, however, this tracer was not suitable for in vivo imaging. Tc99m–labeled oxidized LDL is, taken up by macrophages and has a more rapid blood clearance. It was detected at 150% higher levels in 10 of 11 plaques than in normal arterial segments and therefore provides better image quality.6 Indium In111– or Tc99m–labeled antibodies against plaque and its degradation products, fibrin and platelets, have also been used for imaging of atherosclerosis in an experimental setting.7 In111–labeled polyclonal human immunoglobulin (Ig) G contains an Fc unit that binds to macrophage receptors. It showed a poor target-to-background ratio in rabbits, whereas in humans uptake was found in carotid artery atheromas in 86% of patients, with no correlation with ultrasound findings.8
Platelet and Fibrin Labeling
An unstable plaque with rupture or erosion of the fibrous cap demonstrates activation of platelets and of the clotting cascade. In patients with known cardiovascular events, imaging with In111–labeled platelets correlated with plaque burden and ulcerations seen on ultrasound.9,10 However, blood clearance of labeled platelets is slow, so imaging cannot be performed until 48 hours after the injection. In addition, discontinuation of antiplatelet medication is needed, which cannot be considered in patients with suspected vascular events. Fibrin fragment E1 labeled with iodine I123 and Tc99m can detect thrombi in animals with deep venous thrombosis.11 This agent clears rapidly from the circulation and has good imaging qualities. At present there are no reported studies on the use of this tracer in the evaluation of atherosclerosis.
Smooth Muscle Cell Labeling
In111–labeled Z2D3 is a monoclonal antibody against proliferating smooth muscle cells. Although the specific uptake of this antibody has been demonstrated in a rabbit model12 as well as in human carotid artery plaques,13 it should be emphasized that proliferation of smooth muscle cells is not specifically related to the vulnerable plaque.
Apoptosis Marker Labeling
Apoptosis has been demonstrated in macrophages as well as in smooth muscle cells in atherosclerotic plaques and is often observed in the fibrous cap of a ruptured plaque.14 Annexin V targets phosphatidylserine, a molecule found on the cell membrane during apoptotic cell death. Tc99m–labeled annexin V has been used for specific targeting of active atherosclerotic lesions. Uptake of Tc99m–annexin V has been reported in experimental models of aortic lesions15 as well as in patients with carotid artery disease.16 The role of this substance to assess apoptosis is limited by the lack of specificity. Tc99m–annexin V binds to phosphatidylserine on platelets within the thrombus, but because thrombosis occurs in vulnerable plaques, this feature represents only a relative limitation for imaging with Tc99m–annexin V.2
Peptide Labeling
Peptides are cleared rapidly from the circulation, enabling a better target-to-background ratio. Hardoff et al17 showed significant uptake of a radiolabeled apolipoprotein B analog in experimental atherosclerotic lesions in rabbits. Uptake was also found in carotid lesions in a pilot study in humans.4 Tc99m–labeled synthetic peptides that bind to the glycoprotein (GP) IIb/IIIa receptor on activated platelets is taken up by carotid artery thrombi in dogs18,19 and has been demonstrated in patients with carotid atherosclerosis to have a moderate correlation with ultrasound findings.20 Matrix metalloproteinases (MMPs) are excreted by macrophages and are activated by plasmin as part of the inflammatory process in active atherosclerotic lesions.21,22 Matrix metalloproteinases are responsible for the breakdown of connective tissue in the plaque, leading to instability. Several synthetic radiolabeled inhibitors ofmatrix metalloproteinases have shown promise in plaque imaging in experimental atherosclerosis models.22,23
Fluorodeoxyglucose
Fluorodeoxyglucose (FDG) is an analog of glucose, taken up by and accumulating in cells in proportion to their metabolic activity. FDG imaging is today the “gold standard” in the assessment of patients with cancer, for staging tumors, monitoring response to treatment, and diagnosing recurrence. The transfer of glucose into cells is facilitated by glucose transporters (GLUTs) and sodium glucose cotransporters (SGLTs). After entering the cell, glucose undergoes phosphorylation by hexokinase, forming glucose-6-phosphate. After its glycolysis by hexose-6-phosphate isomerase, fructose-6 phosphate is formed. In contrast, the analog 2-deoxy-D-glucose cannot undergo conversion by hexose-6-phosphate isomerase, and therefore FDG becomes trapped in the cytosol. Glucose and FDG have otherwise similar properties, the rates of phosphorylation in vivo are proportional to each other and reflect the general rates of glucose metabolism.24
Decay of the radioisotope fluorine F18 emits a positron that collides with an electron after travelling a short distance in tissue, generating a pair of 511-keV annihilating photons detected by the PET imaging device. Uptake of FDG labeled with F 18 is usually quantified by means of standardized uptake values (SUVs), which measure the PETtracer uptake within a region of interest in relationship to the injected dose and patient body weight.25 PET provides noninvasive functional information with superior 3- to 5-mm spatial resolution, compared with 1- to 1.5-cm resolution for SPECT. However, both modalities provide very limited morphologic information. Therefore PET (as well as SPECT) is now combined with CT in a single scanner. Hybrid PET/CT and now also PET/magnetic resonance imaging (MRI) improve the sensitivity by anatomic detection of small structures, such as plaque, and may also improve the specificity, confirming active disease in these small lesions.26
Experimental studies in preclinical models documented FDG uptake in animal atherosclerosis.27 Significant FDG activity has been described in the aorta of cholesterol-fed but not chow-fed rabbits, in correlation with macrophage density.27 Increased FDG activity, correlating with macrophage staining and number, was demonstrated in different experimental settings in hypercholesterolemic rabbits and in animals with balloon injury to the aorta.28–30 FDG uptake correlated with vascular inflammation but not with vessel wall or plaque thickness or with smooth muscle cell staining. A correlation between FDG uptake and macrophage infiltration in the aortic wall was found by ex vivo imaging.30 With use of a positron-sensitive fiber optic probe. 4.8-fold higher FDG uptake was demonstrated in rabbits given a high-fat diet in injured iliac arteries, subsequently confirmed to have significantly higher numbers of macrophages and smooth muscle cells, than in normal iliac arteries.31 FDG accumulation within mouse atherosclerotic plaques was found to be six times higher in advanced atherosclerotic lesions than in healthy vessel wall.32
Molecular Imaging of Other Vascular Disease
Similar basic principles are also applicable to other vascular diseases, such as aortic aneurysm and dissection, vasculitis, and vascular graft infection. In addition, molecular imaging modalities are superior to anatomic imaging modalities for follow-up and for early detection of response to therapy.
Fluorodeoxyglucose
There is evidence that vascular FDG uptake is related to levels of inflammatory markers and to the presence of infiltrates in AAAs as well as in other arteries.33 In experimental AAA models in rats, FDG activity correlated with the intensity of CD68+ and ED1 staining, which in turn correlated with activated Tlymphocytes and monocytes-macrophages, respectively.34
Vasculitis.
In vasculitis, molecular imaging techniques can assist in early diagnosis of inflammatory changes in the vascular walls, which are potentially reversible. This inflammatory process precedes anatomic changes, and therefore, at the early stage anatomic imaging modalities are unreliable.
Traditionally, gallium Ga67 citrate and radiolabeled white blood cell (WBC) scans were used to detect vasculitis with either planar or SPECT imaging.35,36 Because Ga67 binds to plasma transferrin and diffuses passively into the inflammatory tissues through the leaky capillaries, it can demonstrate sites of inflammation or infection. However, Ga67 has poor imaging characteristics and delivers a high radiation dose to the patient. On the other hand, radiolabeled WBC scintigraphy is expensive and the labeling of the cells is complex and time consuming. These two tracers are therefore only rarely used in this indication.
FDG imaging reflects the distribution of glucose phosphorylation, which is particularly important in processes with increased glucose metabolism such as inflammation and infection. FDG PET and PET/CT are therefore gaining acceptance in the imaging and early detection of vasculitis.37 In addition FDG PET/CT is being increasingly used to assess response to therapy in documented cases of vasculitis37,38 and has been shown to be superior to anatomic imaging modalities for these clinical indications.
Vascular Graft Infection.
Molecular imaging modalities also have a major role in the assessment of vascular graft infection, and the basic principles already described for other vascular diseases apply also here.
Scintigraphy with Ga67 and labeled WBCs has been used for assessment of suspected prosthetic graft infection. Ga67 scintigraphy was less accurate, so WBC scintigraphy (using cells labeled with either Tc99m or In111) has been the SPECT procedure of choice for detection of infected vascular grafts. WBC accumulation is determined by diapedesis, chemotaxis, and vascular permeability. FDG imaging, however, has several advantages over labeled WBC scintigraphy. Background uptake is low, resulting in relatively high target-to-background ratios, studies are completed within 1 to 2 hours after tracer administration, and there is no need for handling of the blood of potentially infected patients.
Clinical Applications
Molecular Imaging of Atherosclerosis
FDG Uptake in Carotid Plaque
In patients undergoing FDG PET/CT scans for cancer imaging, we and other groups have reported increased FDG uptake in the vascular walls of 31% to 59% of patients older than 50 years,39–41 the uptake being more evident with advancing age, male sex, and the presence of cardiovascular risk factors such as hypertension and hypercholesterolemia.39,40,42,43 Rudd et al44 were the first to show, in a prospective study in eight patients imaged shortly after transient ischemic attack (TIA), that FDG uptake was 27% higher in the symptomatic carotid artery than in the asymptomatic other artery. Autoradiography confirmed the location of uptake in macrophages.44 Co-registration of FDG-PET and MRI indicated the presence of increased tracer uptake in arterial plaques in 10 of 12 patients with an embolic event, consistent with clinical symptoms. In addition, 25% of lesions determined to be nonstenotic by MRI angiography in symptomatic patients had increased FDG activity in significantly inflamed plaques.45 Plaques with unstable features, such as a large lipid core and intraplaque hemorrhage on MRI or echolucency on ultrasound, show higher FDG uptake than more stable lesions.46–49 Lipid-rich necrotic plaques show higher FDG uptake than collagenous or calcified plaques.46 In patients with severe carotid stenosis imaged prior to carotid endarterectomy, an excellent correlation of FDG activity was reported with macrophage staining, but not with plaque thickness, plaque area, or smooth muscle staining, confirming that FDG can be used to assess severity of inflammation in patients with carotid plaques.50,51 Significantly increased carotid FDG activity had been demonstrated in patients with stroke.52,53 FDG uptake in carotid vessels correlates with serum levels of C-reactive protein, a marker of systemic inflammation.54,55 This observation is consistent with the definition of the “vulnerable patient” rather than “vulnerable plaque”: The finding of one vulnerable lesion may increase the likelihood of other vulnerable lesions.27 FDG activity in vessels was greater in patients with more than one cardiovascular risk factor and known coronary artery disease.55 In addition, patients with type 2 diabetes mellitus and/or metabolic syndrome also demonstrated increased carotid FDG uptake.43,56
Monitoring Treatment with FDG Uptake
Sequential PET/CT studies can be used to monitor the effect of lipid-lowering treatment on FDG uptake in atherosclerotic plaques. Reduced plaque inflammation after treatment with probucol, a lipid-lowering antioxidant, has been shown in an experimental rabbit model.57 In human studies, high-resolution MRI has demonstrated that more than 12 months of simvastatin treatment were required to show regression of atherosclerotic plaques.58–60 Of a group of 43 patients with cancer who had increased FDG activity in the thoracic aorta or carotid arteries, half of the subjects subsequently randomly assigned to treatment with simvastatin showed reduced FDG uptake in the arterial wall as well as a decrease in LDL cholesterol by 30% and an increase in HDL cholesterol by 15%. The second half of the study group received dietary management and did not show any change in FDG uptake.60 On the other hand, dietary and lifestyle modifications in asymptomatic subjects caused a 65% reduction in the number of FDG-positive vascular regions.61
FDG-Avid Arterial Wall Calcification
Co-localization of FDG on PET and arterial calcifications on CT has been previously studied using PET/CT scanners, with FDG-avid arterial wall calcifications observed in 2% to 14% of lesions.39–41 Findings in the vascular wall of patients more than 50 years of age showed three different patterns: PET-negative and CT-positive, PET- and CT-positive, and PET-positive and CT-negative. Although there was a large number of CT-positive atherosclerotic lesions (in 82%-92% of patients), FDG uptake indicating active inflammation was variable (15%-74%). Lesions with evidence of both past (C-positive) and current (PET-positive) inflammation were seen in 10% of patients.40,62 Other researchers have described no or a negative correlation between areas of calcification and FDG uptake.63 The incongruence between FDG-PET and CT findings may represent variations in the stage of the atherosclerotic process. The presence and extent of calcifications on CT define morphologic changes likely consistent with advanced disease, whereas FDG avidity demonstrates transient plaque inflammation, possibly indicating future complications.
Change in Plaque FDG Uptake Over Time
Changes in vascular wall uptake over time in 50 patients with cancer and a mean age of 68 years who had repeat PET/CT scans 8 to 26 months apart were also reported. Changes in the pattern of vascular findings were observed in 48% of FDG-positive sites, compared with 4% of PET-negative foci and 7% of CT-positive lesions (Fig. 25-1).62 Transient FDG uptake in the arterial wall was found in a group of 205 studies from 50 patients with a mean age of 49 years who underwent at least four PET/CT scans for a mean follow-up of 27 months.63 The variable vascular wall FDG uptake suggests that inflammation may be a transient feature of a dynamic process, whereas calcifications may represent a chronic process or stable atherosclerosis.62 This observation may have potential future implications for the design of trials for monitoring response of vascular inflammation to therapeutic interventions.
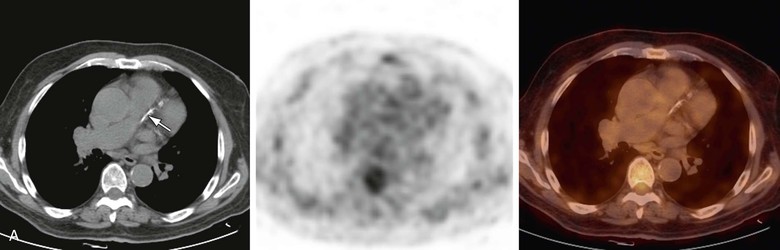
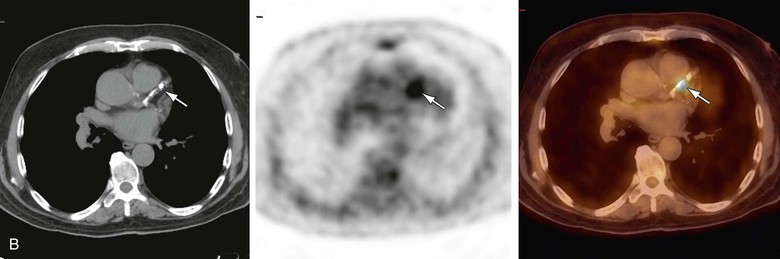
Figure 25-1 Changing pattern of vascular wall fluorodeoxyglucose (FDG) uptake in 70-year-old patient referred for routine follow-up after completion of chemotherapy for diffuse large B-cell lymphoma. Whole-body FDG PET/CT images showed no evidence of FDG-avid disease. A, Selected axial slice shows left anterior descending (LAD) coronary artery calcifications on CT (left) with no focal increased FDG activity on PET (center) or on the fused PET/CT image (right). B, On follow-up scanning 8 months after the previous study, CT shows that LAD calcifications have progressed (left), and there is also focal FDG activity in the LAD calcifications on the PET (center) scan and localized activity on the fused PET/CT scan (right).
Other Tracers for Plaque Imaging
The majority of data related to metabolic imaging of atherosclerosis refers to the use of FDG, mainly because of its wide availability and the vast clinical experience with its use accumulated over the last few decades. Because of the limitations of FDG, including the nonspecific nature of its uptake in various types of cells that metabolize glucose as well as the technically challenging imaging of the coronary vessels, other tracers are being sought and evaluated. Preliminary work using C11-PK11195, a selective ligand of a translocator protein with 20 times greater density expression on macrophages than on vascular smooth muscle cells, has been reported. This tracer binds to macrophage-rich regions in human carotid plaques,64 and promising results have been reported in patients with vasculitis.65
Other potentially useful tracers include Ga68 DOTATATE, which binds to somatostatin receptors subtype 2 expressed by macrophages,66 and carbon C11– or F18–labeled choline, which is taken up by macrophages, undergoes phosphorylation and metabolism to phosphatidyl choline, and is incorporated into the cellular membrane.67,68 In an experimental model, F18–labeled choline uptake correlated better than FDG uptake with fat staining and macrophage-positive areas.68 In patients with cancer, C11–labeled choline was not taken up by the normal vascular wall or by calcified vascular wall lesions.67
Active plaque calcification can contribute to plaque instability69 and can be assessed with F18–labeled sodium fluoride (NaF), which replaces hydroxyl groups on hydroxyapatite molecules. NaF can potentially better assess the coronary vessels than FDG owing to the lack of significant myocardial uptake of NaF and its high target-to-background ratio. A group of 45 patients with cancer were assessed with FDG, NaF, and CT for the presence of inflammation, active calcium deposition, and calcifications, respectively. Of 105 lesions with NaF uptake, 77% showed calcification on CT and 14% showed increased FDG activity.70 It has been hypothesized that in early atherosclerosis, only inflammatory changes and therefore only FDG uptake are present, whereas later during the course of this process, there is also active calcification, reflected by uptake of both FDG and NaF. At a late stage, when the density of calcium deposits exceeds a certain threshold, calcifications would also become visible on CT. When calcification and mineralization exceed the inflammatory process, uptake of NaF but not of FDG would be found in calcified lesions seen on CT. At the final stage of dense calcification with no significant calcium turnover, lesions would be seen only on CT.27
Molecular Imaging of Aortic Pathology
Abdominal Aortic Aneurysm
Increased FDG activity has been reported in patients with asymptomatic abdominal aortic aneurysms (AAAs), probably owing to the presence of transmural inflammation and the accumulation of FDG in macrophage aggregates.71,72 A possible association between increased FDG uptake and AAA expansion and rupture has been suggested.73 Higher FDG in AAA uptake has been associated with higher wall stress74 and correlated with instability and macrophage infiltration.33 Aneurysmal dilatation develops after an inflammatory phase with increased FDG activity.75 An inverse relationship between FDG uptake and future expansion of the aneurysm, as measured by Doppler ultrasound, was found in 25 patients with AAAs who underwent routine surveillance over a period of 12 months.76 In patients with asymptomatic AAAs, no increase in FDG uptake was observed in large lesions referred to surgery.77 A marked reduction in cell density in AAAs correlated with low FDG activity.77 Increased FDG activity is more frequent in small aneurysms and in lesions with inflammatory changes, which are more often symptomatic and require direct surgical intervention (Fig. 25-2).33
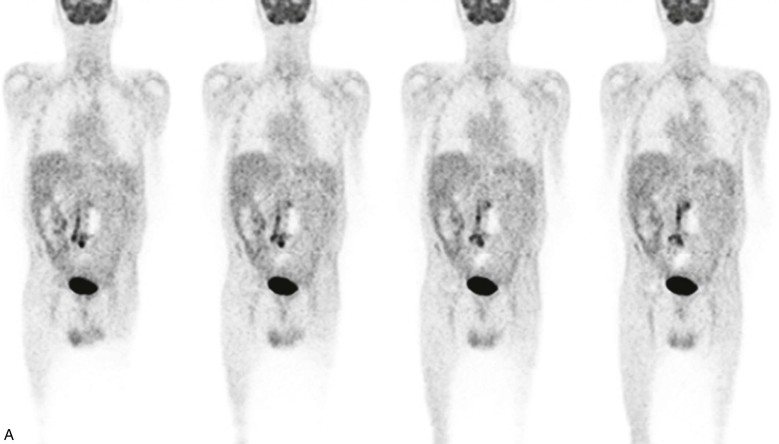
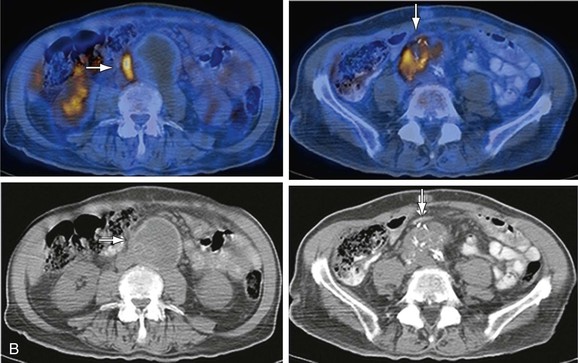
Figure 25-2 Fluorodeoxyglucose (FDG) uptake in an infected graft inserted in an abdominal aortic aneurysm (AAA). The patient, a 79-year-old diabetic man, had undergone surgical repair of a dissecting AAA and implantation of a bi-iliac vascular graft 10 years prior to the current examination. He presented with severe abdominal pain. CT angiography demonstrated a thickened posterior wall of the aneurysm. A, Selected coronal PET slices demonstrate a linear area of intense abnormal FDG uptake in the lower abdomen and upper pelvis. B, The uptake is localized by PET/CT at its upper level to a thickened AAA wall (left column, arrows), consistent with active inflammation, and at the pelvic level to a soft tissue mass surrounding the right component of the graft and surgical clips (right column, arrows), consistent with an infected right vascular graft.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


