(1)
Department of Neuroscience, University of Turin Ospedale Molinette, Turin, Italy
Abstract
Rokitansky is reported to be the first to have described this kind of pathology which he called “vascular brain tumor in pial tissue” (Rokitansky 1846). It was Virchow (1862–1863) who first differentiated tumors from brain angiomas, which were identified as vascular malformations of congenital derivation. The concept that brain arteriovenous malformation (BAVM) is an anomaly caused by errors during vascular development in the embryo was suggested by Cushing and Bailey (1928) and Dandy (1928). However, some difficulties in the differential diagnosis between BAVMs and tumors remained, as noted by Zülch (1957) and Russell et al. (1959). An accurate description of this pathology as a definite congenital malformation was proposed by McCormick (1966). The same author made a classification, which, with some modification (Challa et al. 1995; Yaşargyl 1987, 1999; Chaloupka and Huddle 1998; Valavanis et al. 2004), is still valid today.
12.1 Introduction
Rokitansky is reported to be the first to have described this kind of pathology which he called “vascular brain tumor in pial tissue” (Rokitansky 1846). It was Virchow (1862–1863) who first differentiated tumors from brain angiomas, which were identified as vascular malformations of congenital derivation. The concept that brain arteriovenous malformation (BAVM) is an anomaly caused by errors during vascular development in the embryo was suggested by Cushing and Bailey (1928) and Dandy (1928). However, some difficulties in the differential diagnosis between BAVMs and tumors remained, as noted by Zülch (1957) and Russell et al. (1959). An accurate description of this pathology as a definite congenital malformation was proposed by McCormick (1966). The same author made a classification, which, with some modification (Challa et al. 1995; Yaşargyl 1987, 1999; Chaloupka and Huddle 1998; Valavanis et al. 2004), is still valid today.
12.2 Classification
Arteriovenous malformation (AVM)
Vein of Galen AVM
Cavernous malformations (cavernomas)
Capillary malformations (telangiectasias)
Developmental venous anomaly (DVA), venous angiomas
Transition forms
Vascular malformations part of well-defined congenital-hereditary syndromes
Rendu–Osler syndrome
Sturge–Weber syndrome
Wyburn–Mason syndrome
Klippel–Trenaunay–Weber syndrome
12.3 Arteriovenous Malformations
12.3.1 Pathogenesis and Pathology
The certain pathogenesis of AVMs is not clear. They are considered to be congenital malformations. The embryological development of cerebral vessels occurs in two phases: vasculogenesis and angiogenesis. In the vasculogenesis, angioblasts differentiate into endothelial cells to form the primary vascular plexus. Later, angiogenesis follows, in which the primary plexus undergoes remodeling and organization, leading to the formation of the final cerebral vessels (Streeter 1918; Risau and Flamme 1995; Risau 1997). The causes of an aberrant vasculo-angiogenesis leading to AVMs are unknown. Many factors are probably involved; among them, some endothelial growth factors (VEGFR1-VEGFR2) and their binding receptors (FLt-1; FLk-1) have been identified as important for the normal development of cerebral vessels. Absence, mutation, or highest levels of these factors could lead to aberrant development and formation of AVMs (Shalaby et al. 1995; Fong et al. 1995; Sonstein et al. 1996; Uranishi et al. 2001; Hashimoto et al. 2001).
When considering the embryological development of the cerebral arteries and veins, some authors (Mullan et al. 1996a, b) have suggested that AVMs could already be present before the third month of gestation. In some cases, an AVM may be relatively small at birth and grow later. There are, however, reports describing the appearance of cerebral AVMs later in life among patients in whom previously performed magnetic resonance imaging (MRI) showed no malformations. In some of these patients, cerebral AVMs occurred in the pathologically altered brain as a result of different causes, such as vascular pathology (Schmit et al. 1996; Song et al. 2007), heterotopia (Stevens et al. 2009), and changes after radiosurgery (Rodriguez-Arias et al. 2000); in others, the brain parenchyma was completely normal (Gonzalez et al. 2005; Bulsara et al. 2002). These observations raise doubts about the congenital nature of cerebral AVMs, which—at least in some cases—seem to be acquired lesions caused by different nonspecific insults on the brain.
The main angioarchitectural characteristic of an AVM is an area called the nidus, in which a direct shunting between arteries and veins occurs without interposed capillaries. The elevated intravascular flow leads to changes of the vessels. Histology shows the nidus to be composed basically of dilated arteries and veins. In some vessels, the wall structure is still recognizable, characterized by the presence of a media with smooth muscle cells and an elastic lamina in the arteries and an absence of muscle cells in the veins. In other arteries, prominent changes, characterized by areas of wall thickening caused by proliferation of fibroblasts, muscle cells, and an increase in connective tissue, are present. Segments with a thinning of the wall also occur, which potentially can lead to aneurysm formation. Severe changes take place in the venous sector, forming so-called arterialized veins, characterized by wall thickening, which is particularly due to fibroblast proliferation, not smooth muscle cells. The interposed parenchyma shows gliosis, hemosiderin pigmentation, and calcifications, resulting from ischemia or previous hemorrhages. The surrounding parenchyma may appear normal or show similar changes (Challa et al. 1995; Kalimo et al. 1997; Brocheriou and Capron 2004).
12.3.2 Incidence
The incidence of AVMs is not completely known. In general autopsy, they are discovered with a frequency of 0.15–0.8 % (McCormick 1984; Jellinger 1986). Multifocal lesions can occur with a frequency of 1–10 % (Perret and Nischioka 1966; Rodesch et al. 1988; Willinsky et al. 1990); the latter are more frequent in pediatric patients, where they are reported to be twice as common as in adults (Rodesch et al. 1988; Lasjaunias 1997).
12.3.3 Clinical Relevance
Of AVMs, 5–10 % remain asymptomatic and are diagnosed incidentally by CT or MR investigations performed for other reasons. Some 40–50 % present with intracranial hemorrhage, 30 % with seizures, 10–15 % with headaches, and 5–10 % with neurological deficits (Perini et al. 1995; Stapf et al. 2002; Hofmeister et al. 2000; Valavanis et al. 2004); the incidence of symptomatic cerebral malformation in the adult population is reported to be one-tenth the frequency of intracranial aneurysm (Berenstein and Lasjaunias 1992; Valavanis et al. 2004). The most important risk in AVM is hemorrhage, which is calculated to be 2–4 % per year, with an annual rate of mortality of 1 % and severe morbidity of 1.7 % (Graf et al. 1983; Crawford et al. 1986; Ondra et al. 1990; Mast et al. 1997). The risk of a repeated hemorrhage after an initial episode is reported to increase in the first year, later decreasing until it reaches the level of the initial risk (Graf et al. 1983; Mast et al. 1997). It is the most frequent initial symptom in children (Berenstein and Lasjaunias 1992; Rodesch et al. 1995; Lasjaunias 1997).
Cases of spontaneous thrombosis of AVMs (Sukoff et al. 1972; Levine et al. 1973; Mabe and Furuse 1977; Pascual-Castroviejo et al. 1977; Sartor 1978; Nehls and Pittman 1982; Omojola et al. 1982; Wakai et al. 1983; Pasqualin et al. 1985; Barker and Anslow 1990; Ezura and Kagawa 1992; Hamada and Yonekawa 1994; Abdulrauf et al. 1999) as well as its possible recanalization occurring even a few years later (Mizutani et al. 1995) have been reported. A long follow-up of these patients is mandatory.
12.3.4 Location
The majority of AVMs (85 %) are located in the supratentorial area, and only 15 % are infratentorial (Perret and Nischioka 1966; Yaşargyl 1999). Supratentorial AVMs can be further divided (Valavanis et al. 2004): neopallial, including AVMs in the frontal, parietal, temporal, and occipital lobes and corpus callosum, and archi- and paleopallial, including those in the limbic and paralimbic system (amygdala, hippocampal, parahippocampal, septal, gyrus cinguli, and insular AVMs). AVMs can be located in a sulcus (sulcal), gyrus (gyral), or both (sulco-gyral). They can remain superficial or extend deeply toward the ventricle, basal ganglia, and thalamus. AVMs involving primary deep structures or ventricles are rarer. They are more frequent in pediatric patients (Berenstein and Lasjaunias 1992).
Infratentorial AVMs can be divided into those involving the cerebellum (hemisphere, vermis), located on the superior – inferior convexity or on its anterior surface. Deep structures can be primarily involved or be an extension of a superficial lesion. Primary AVMs in the brainstem are very rare, as are those of the fourth ventricle (Garcia Monaco et al. 1990; Liu et al. 2003).
12.3.5 Diagnosis
MRI, including functional studies, provides informations about the site and extension of AVMs. Furthermore, it shows which functional changes have occurred in the affected and unaffected hemisphere (Alkadhi et al. 2000). Angiography is essential in defining the angioarchitecture of the malformation. It comprises selective angiography of the internal and external carotid arteries and the vertebral artery, followed, when necessary, by super-selective examinations aimed to characterize the supplying arteries, venous drainage, and aspects of the nidus.
12.3.5.1 Supplying Arteries (Feeders)
These can be fairly dilated and tortuous, unique or multiple, and arise from one or more vascular territories. Cortical branches are involved in superficial AVMs (Figs. 12.1, 12.2, 12.4, 12.6, and 12.12). Perforators (deep and medullary arteries) and choroidal arteries can be recruited every time deep structures and ventricles are primary or secondary involved by large cortical AVM extending to the depth (Figs. 12.3a–e, 12.7, and 12.9).
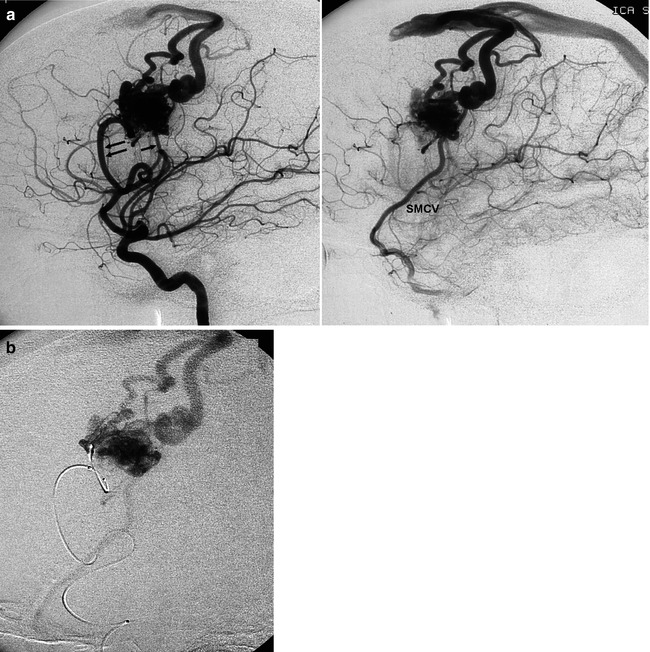
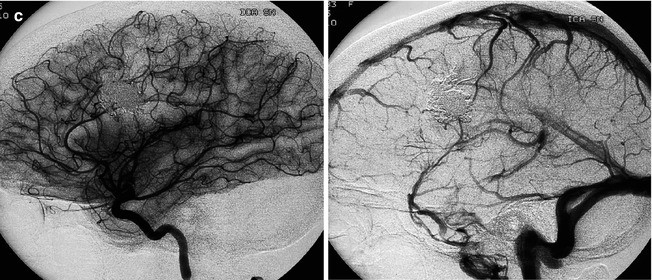
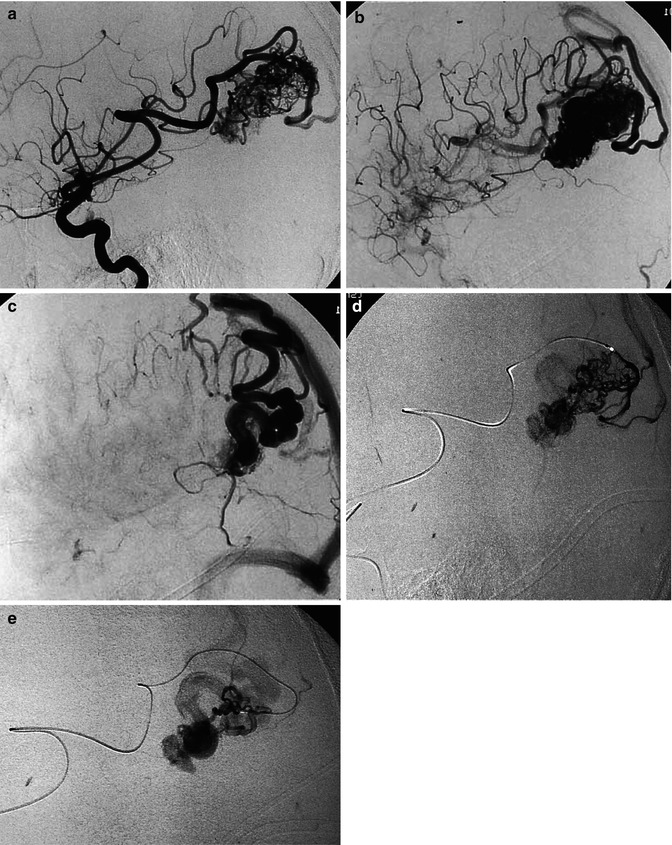
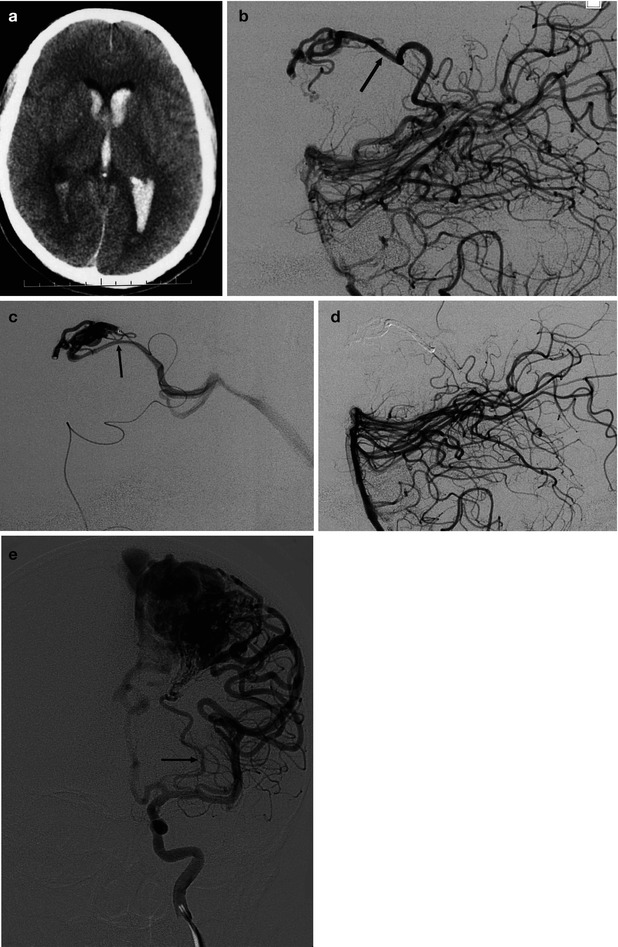
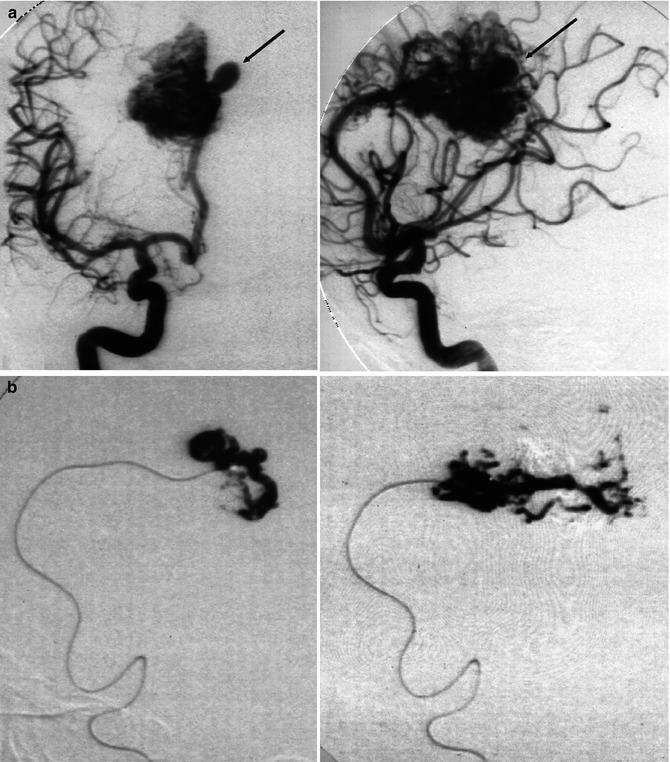
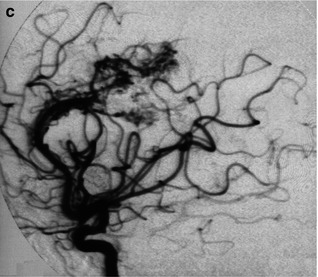


Fig. 12.1
Well-defined nidus of lateral frontal AVM presenting with epilepsy. Lateral angiogram, early and late phases (a). The AVM is supplied by a dilated insular branch (double arrow). A second, smaller feeder appears posteriorly (arrow). Cortical drainage in the superior sagittal sinus, with partial retrograding injection of the anterior segment, and inferiorly into the superficial middle cerebral vein (SMCV). (b) Super-selective catheterization preceding embolization with Onyx. (c) Lateral angiogram, arteriovenous phase performed 2 months after complete occlusion of the AVM, showing normalization of the arteries and draining veins

Fig. 12.2
Laterotemporal occipital AVM, presenting with hemorrhage, supplied by distal branches of the gyrus angularis artery. Carotid angiogram, lateral view, arterial (a) and venous phases (b, c). There is a different venous drainage related to the corresponding compartments. These are well demonstrated on super-selective studies (d, e). At the periphery of the nidus, an isolated arteriovenous shunt is recognizable (d)

Fig. 12.3
(a–d) AVM in young patient presenting with hemorrhage involving the third and lateral ventricles. (a) CT showing the hemorrhage. (b) Lateral vertebral angiogram. There is a dilated posterior medial choroidal artery (arrow) supplying the AVM in the roof of the third ventricle. (c) Selective study showing nidus of the AVM and drainage in the internal cerebral vein (arrow), continuing into the Galen vein and straight sinus. (d) Control angiogram after endovascular treatment with occlusion of the AVM with acrylic glue. (e) Another example of a large parietal AVM with involvement of an enormously enlarged perforator branch (arrow) of M1. The perforator has a common origin with a distal cortical branch


Fig. 12.4
AVM involving the corpus callosum and adjacent gyrus cinguli presenting with hemorrhage. (a) Internal carotid angiogram (AP, lateral view) showing the compact nidus supplied by the pericallosal artery. In the posterior medial part of the nidus, a dilated vascular structure is recognizable (arrow). It is not possible to determine whether this corresponds to a nidal aneurysm or a pseudovenous aneurysm. (b) Two selective studies of branches of the pericallosal artery preceding injection of acrylic glue aimed to occlude partially the nidus and especially the aneurysm. (c) Control angiogram post treatment, well tolerated by the patient, who was operated on 1 month later with finally clinically good results
Each feeder can end in the nidus, connected through one or more small branches with one or more venous channels, in various combinations (Houdart et al. 1993), forming what is termed the plexiform aspect of the nidus (Figs. 12.1 and 12.2). Otherwise, after giving branches to the AVM, the feeders continue distally to supply the normal parenchyma. On an angiogram, they appear to end in the nidus, though they do in fact run further distally. The distal part, however, is not always recognizable, owing to the steal phenomenon present in the nidus. In other cases, a large artery “en passage feeder” running adjacent to the nidus can give some small branches to the nidus, coursing further to the normal parenchyma (Figs. 12.5a and 12.6). All these aspects should be carefully studied with selective injections since embolization of these feeders carries the risk of ischemia of the normal parenchyma (Berenstein and Lasjaunias 1992; Valavanis 1996; Chaloupka and Huddle 1998; Pierot et al. 2004; Valavanis et al. 2004).
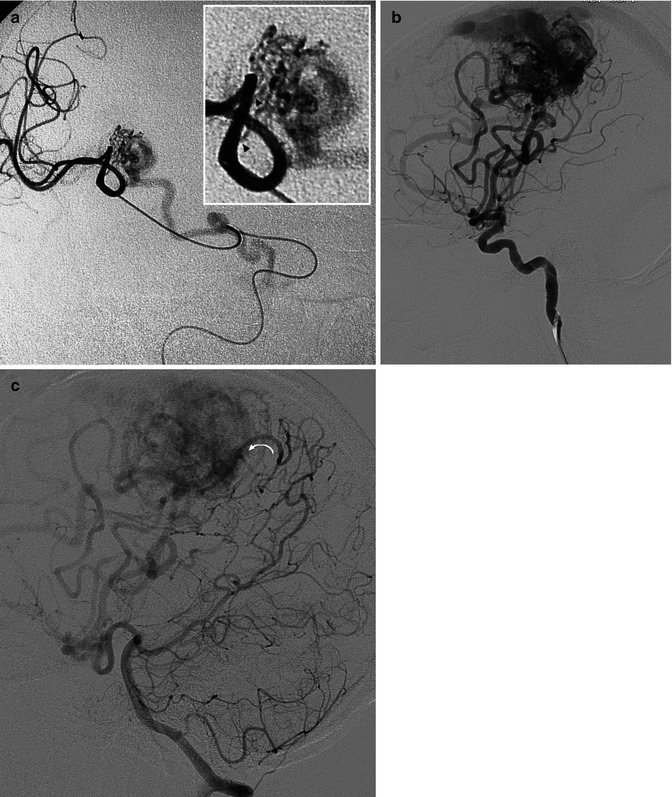
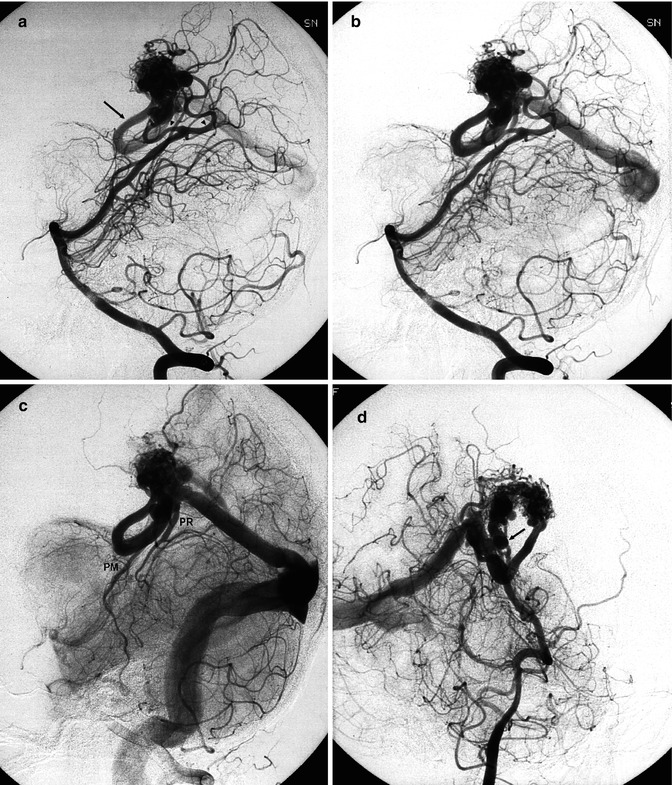
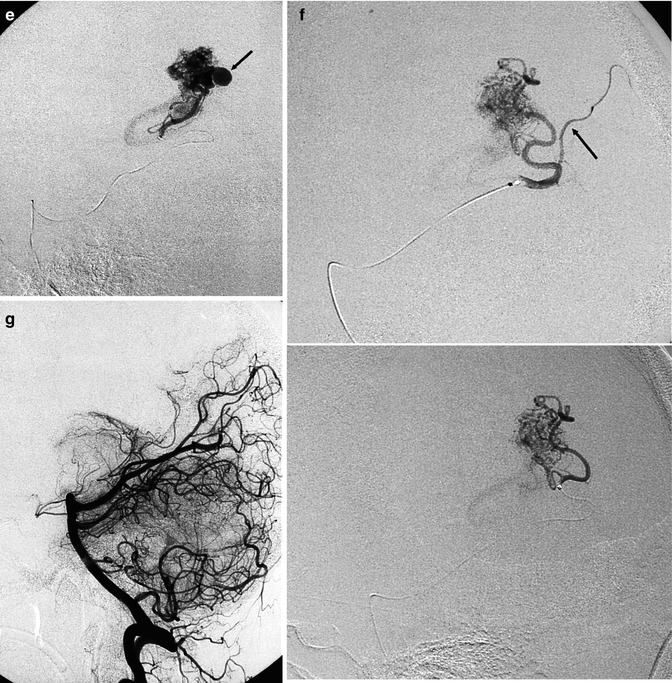

Fig. 12.5
(a) Example of “en passage feeder”. From a proximal part of a branch of MCA arise small branches (arrow-head) supplying a temporo-insular AVM. (b, c) Very large parietal AVM supplied by branches of ACA and MCA. (b) Carotid angiogram. (c) Vertebral angiogram. Indirect involvement of distal branches of PCA through opening of leptomeningeal anastomosis between PCA and MCA. One of the branches (arrow) is very enlarged


Fig. 12.6
Patient with large hematoma located in the left deep medial occipital retrosplenial area, removed in the acute phase. After clinical improvement, vertebral angiography (a) showed the AVM supplied by two feeders (arrowhead) arising from the P4 segment of the left PCA. Drainage (b) into a large medial atrial vein (arrow), continuing into the Galen vein and straight sinus. Owing to hypertension (c) in the Galen vein, there is a retrograde injection of the precentral vein (PR) and posterior mesencephalic vein (PM). There is a proximal duplication of the straight sinus. In the oblique view (d), a second smaller drainage (arrow) is visible, also entering the Galen vein. Catheterization of the branch (e) supplying the compartment with intranidal aneurysm (arrow). Catheterization of the second branch (f) with a progressive advance of the microcatheter distal to a normal parenchymal branch (arrow). Posttreatment angiogram (g). The remaining minimal component of the AVM supplied by the pericallosal artery was treated by radiosurgery
Sometimes, indirect feeders can reach the nidus through the opening of leptomeningeal (pial) anastomoses (Fig. 12.5b, c). This occurs when an important branch supplying the AVM ends completely in the nidus and no branches reach the distal normal parenchyma, which is supplied indirectly by the collateral circulation. The latter can extend to the AVM and supply its distal part (Berenstein and Lasjaunias 1992; Chaloupka and Huddle 1998; Valavanis et al. 2004).
Involvement of meningeal branches is reported in about 30 % of cases (Newton and Cronquist 1969; Rodesch and Terbrugge 1993). This occurs through anastomoses between the meningeal arteries and the pial branches involved in vascularization of the AVM. In this context, it should be remembered that dilated dural branches can be a cause of headache. Furthermore, in selected cases, the dural branches can be catheterized and used to reach the nidus of the malformation and inject embolic material.
Finally, an interesting aspect, occurring in the cerebral arteries, as well as in the branches of ECA when involved, and in the veins, is their dilatation due to the increased in–out flow, which disappears with return to normalization, when the vascular malformation is eliminated. This is due to the specific characteristic of the vessels to adapt to the different vascular conditions.
12.3.5.2 Aneurysms
These can be located far from the nidus on one or more supplying arteries. They are thought to be due to the increased flow (flow-related or stress aneurysm) and frequently, though not always, disappear when the AVM is excluded (Berenstein and Lasjaunias 1992; Valavanis and Yaşargil 1998). They can be the cause of subarachnoid or parenchymatous hemorrhage (Stapf et al. 2006). The frequency of aneurysms is reported to increase with the age of the AVM (Berenstein and Lasjaunias 1992). This probably means that the development of these aneurysms is due to the high flow associated with the AVM, but it is also the result of the chronicity of the shunt (Valavanis 1996). In our experience, the majority of these aneurysms occur in old patients, especially in the vertebrobasilar sector (Figs. 11.13, 12.13, 12.14, and 12.16). Rarely, aneurysms can be found on an arterial branch independent of the AVM. The pathogenesis of these is probably the same of the other aneurysms, as described in Sect. 11.4.
Other small aneurysms are located near or within the nidus (intranidal aneurysms). These can be better identified by selective studies. They are very frequent and are thought to be responsible for hemorrhage in many cases (Willinsky et al. 1988; Marks et al. 1992; Turjman et al. 1994; Pollock et al. 1996; Redekop et al. 1998; Bradac et al. 2001; Pierot et al. 2004; Valavanis et al. 2004) (Figs. 12.4, 12.6, 12.7, and 12.11).
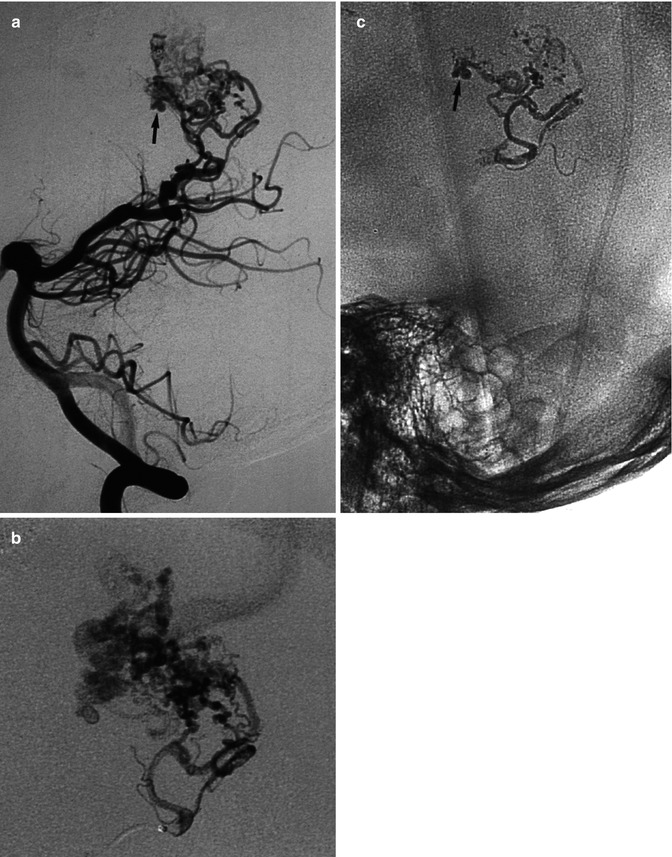

Fig. 12.7
Example of an intranidal aneurysm, probably responsible for repetitive small intraventricular hemorrhage in a patient with a very large right parietal AVM extending deeply toward the lateral ventricle. Lateral vertebral angiogram (a) showing part of the AVM with an intranidal aneurysm (arrow) in the vascular territory of the medial posterior choroidal artery. Selective study (b) preceding injection of acrylic glue. Cast of the glue (c) involving part of the nidus and also the aneurysm (arrow). Despite only partial treatment, the hemorrhagic episodes arrested completely over a period of many years
One notable type is the pseudoaneurysm, which develops at the site of rupture of the AVM; these are detected in patients presenting clinically with recent AVM rupture (Valavanis et al. 2004). Pseudoaneurysms lack a true vessel wall and consist of a pouch arising from a partially reabsorbed hematoma. They can be angiographically identified by their irregular shape and location at the margin of a recent hematoma (Berenstein and Lasjaunias 1992; Garcia Monaco et al. 1993; Valavanis 1996; Valavanis et al. 2004) (Fig. 12.9).
12.3.5.3 Other Changes
Among other changes of the supplying arteries, there is stenosis, which is commonly due to intrinsic changes in the wall and is characterized by intimal hyperplasia, mesenchymal proliferation, and capillary proliferation through the adventitia (Willinsky et al. 1988) (Fig. 12.11). Moyamoya pattern at the base of the brain has also been reported, probably being the result of hemodynamic stress (Mawad et al. 1984; Berenstein and Lasjaunias 1992).
12.3.5.4 Venous Drainage
The type of drainage commonly depends on the location of the AVM and is thus predictable. It can, however, be aberrant due to preexistent variants or the formation of a collateral circulation following occlusion or stenosis in the venous sector; it may be a venous adaptation in an attempt to reduce the high intranidal pressure. Venous drainage can be superficial, deep, or both, and it consists of a single draining vein or several venous channels (Figs. 12.1, 12.2, 12.6, 12.8, 12.9, and 12.12). In the latter case, a specific venous drainage can be seen after injection of each correspondent supplying artery. In other cases, the same venous drainage is recognizable after injecting different feeders. When several venous channels are involved, it is a multi-compartimental AVM; where there is just a single draining vein, it is a unique-compartment AVM (Yaşargyl 1987; Berenstein and Lasjaunias 1992; Valavanis et al. 2004). In this context, it should be considered that multiple venous drainage can be only apparent owing to the fact that the unique draining vein divides early into more veins.
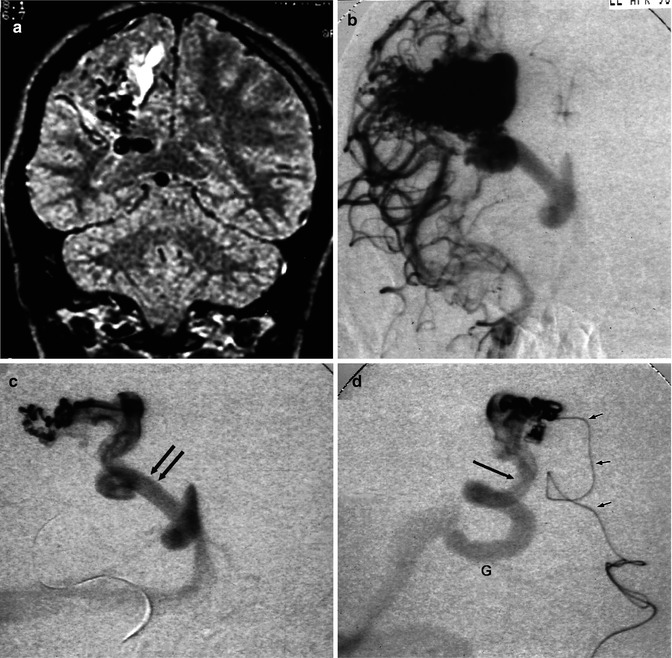
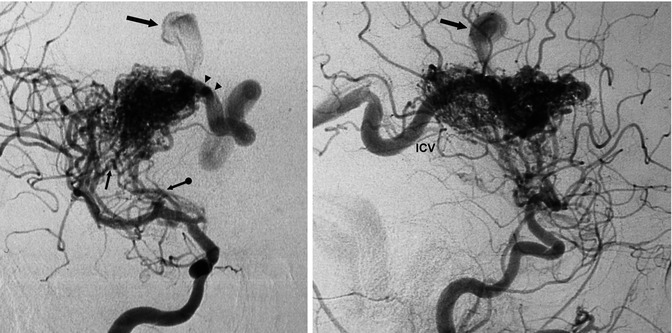

Fig. 12.8
Parietal AVM. (a) Coronal MRI T2-weighted image showing the extension of the lesion toward the ventricle. There is gliosis due to a previous hemorrhage. (b) Carotid angiogram, AP view, showing the compact nidus and deep venous drainage. (c) AP view, during selective study. The drainage occurs through a very dilated medullary vein, continuing into the medial atrial vein (arrows) entering the Galen vein. (d) Lateral view, corresponding to the image in (c). Microcatheter (small arrows). Dilated medial atrial vein (arrow) draining into the Galen vein (G)

Fig. 12.9
AVM in a young patient presenting with hemorrhage involving the basal ganglia and white matter. Carotid angiogram, AP and lateral views. The AVM is supplied by dilated perforators (arrow with dot) and by several branches arising from the M2 segment of the MCA (arrow). The drainage occurs in the thalamostriate vein (arrowhead), continuing into the internal cerebral vein (ICV). There is another partially injected venous pouch (large arrow), which probably corresponds to a pseudoaneurysm. The patient underwent operation
The veins draining the AVM are always dilated. The dilatation is sometimes enormous, forming large pouches (Fig. 12.12) which can be the result of distal stenosis or thrombotic occlusion. The cause of the stenosis may differ. It can be due to hyperplasia of the wall components as a reaction to the increased flow and pressure. Otherwise, the stenosis occurs when the vein enters the dura or may be the result of a kinking of an ectatic vein or bone compression. Sometimes, these pouches result from pseudoaneurysms, as described in Sect. 12.3.5.2 (Fig. 12.9). Some aspects of the venous drainage (unique veins, deep venous drainage, stenosis, and large pouches) are considered potential risks or may already be the cause of a present hemorrhage (Vinuela et al. 1985, 1987; Berenstein and Lasjaunias 1992; Turjman et al. 1995; Muller-Forell and Valavanis 1996; Pierot et al. 2004; Valavanis et al. 2004).
12.3.5.5 Nidus
The extension of the nidus varies from very large to very small. Small AVMs have a greater tendency to rupture (Fig. 12.10) (Graf et al. 1983; Pierot et al. 2004). The same is true for deeply located and posterior fossa AVMs (Figs. 12.3, 12.6, 12.7, and 12.9). Some authors (Garcia Monaco et al. 1990; Berenstein and Lasjaunias 1992) reported a higher tendency for hemorrhage also in temporo-insular and callosal AVMs (Fig. 12.4). The nidus can be mono- or multicompartmental (Fig. 12.2). It is interesting to note that with increasing experience in vascular treatment, small connections through the different compartments may become recognizable, and so the slow injection of embolic material can penetrate completely the nidus. In this context, it is possible for numerous small supplying branches to arise from a large main feeding artery. During the injection of embolic material into the nidus through one of the small branches chosen, a retrograde injection of another arterial feeder can occur. This should be immediately recognized to avoid retrograde injection also of the main feeder.
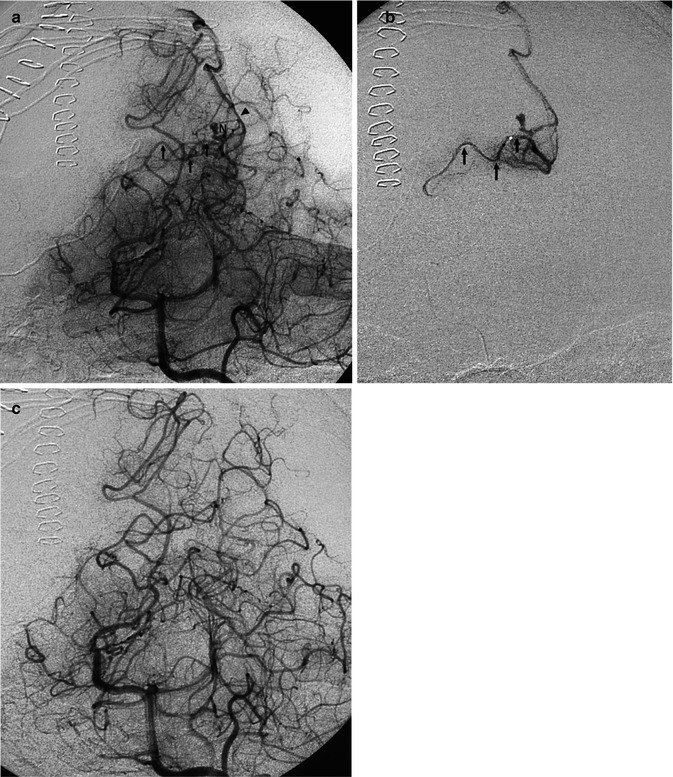

Fig. 12.10
Example of a very small medial occipital AVM presenting with hemorrhage. Vertebral angiogram, oblique view. (a) Feeding artery (arrows) arising from the P4 segment of the PCA. Small nidus (N). Unique draining vein (arrowhead). Super-selective study (b) preceding occlusion with acrylic glue. Control angiogram (c) post treatment
The presence of an intranidal aneurysm has already been described. Arteriovenous shunts can be very large, leading to the formation of large fistulas, characterized on an angiogram by an immediate injection of the venous sector. The fistula can be the unique feature of the AVM or only part of the plexiform nidus (Fig. 12.12) (Berenstein and Lasjaunias 1992; Chaloupka and Huddle 1998; Valavanis et al. 2004). The fistulas are more frequent in children (Rodesch et al. 1995; Lasjaunias 1997).
Finally, the nidus can be well defined (compact nidus) (Figs. 12.1, 12.2, and 12.4) or without precisely identified borders (diffuse nidus) (Fig. 12.11). In the latter condition, the feeders are numerous, not particularly dilated, and without a specific dominant sector. The veins are only moderately dilated with relatively slow flow. The nidus is large, involving frequently more lobes. Endovascular as well as surgical treatment is particularly difficult or impossible (Yaşargyl 1987; Berenstein and Lasjaunias 1992).
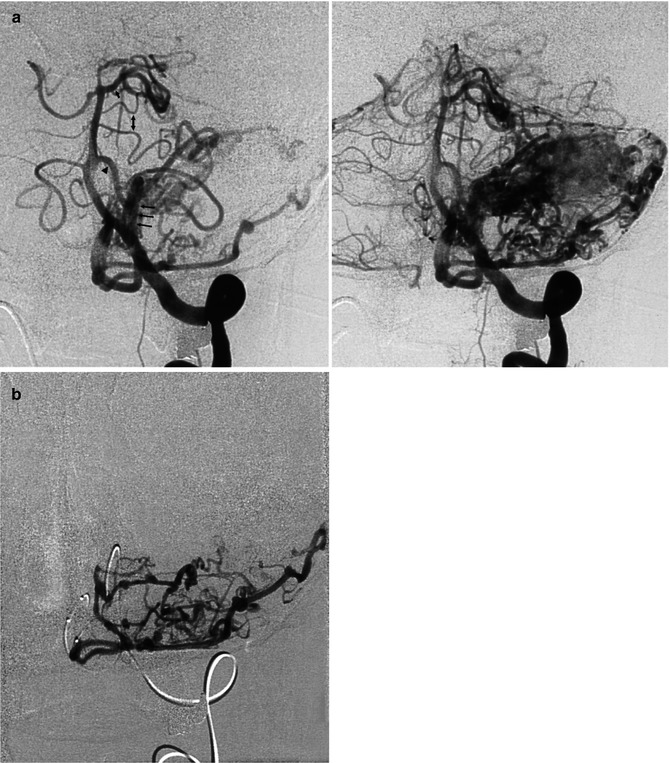
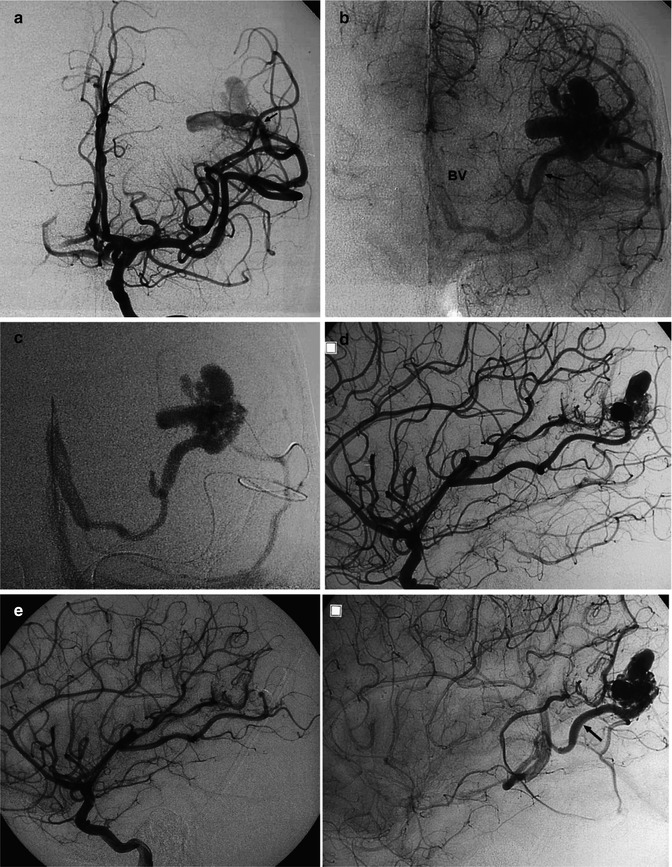
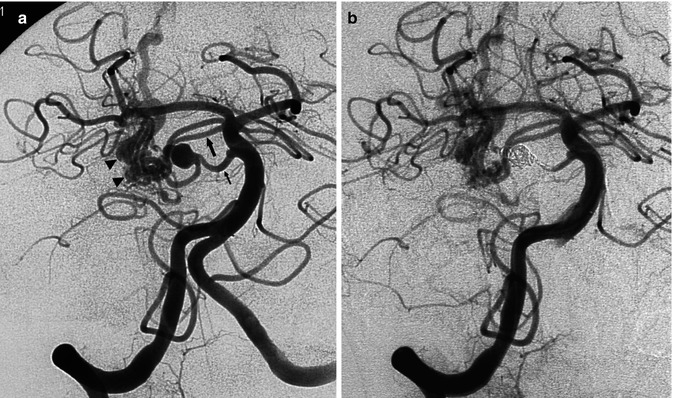
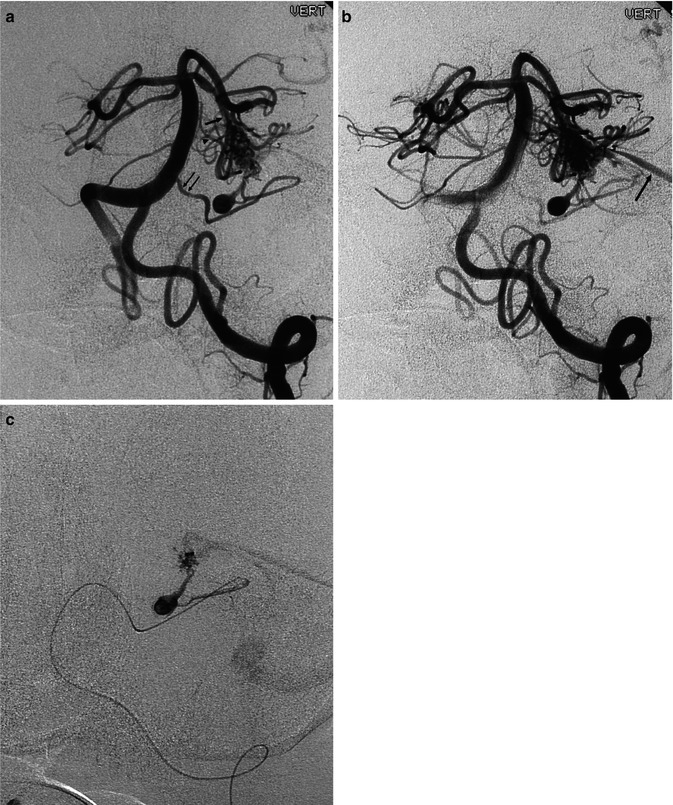
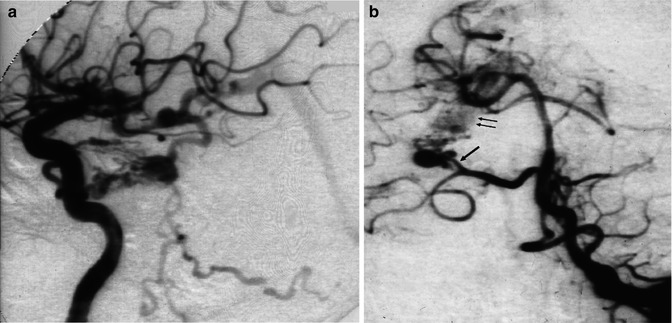
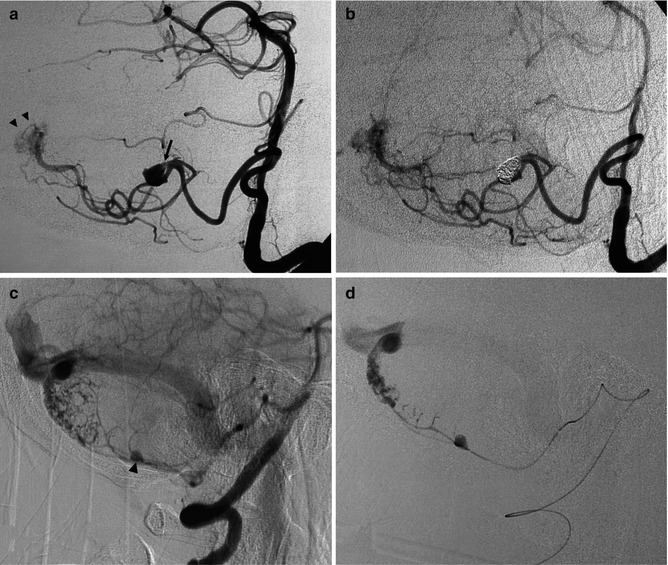
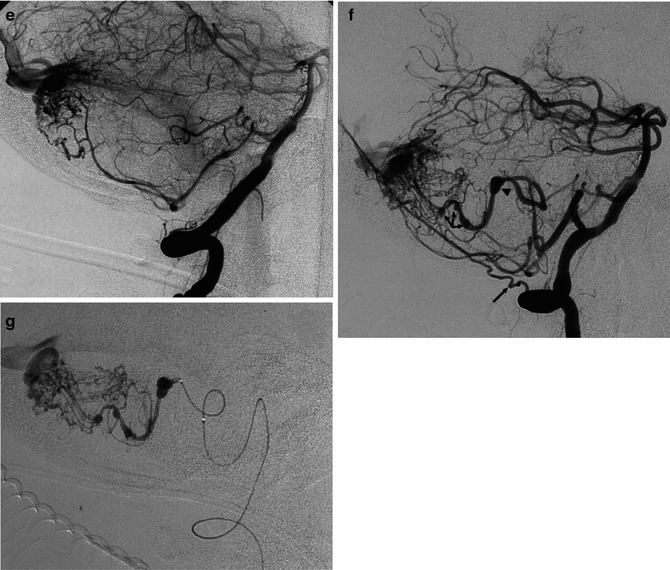

Fig. 12.11
AVM with a diffuse character, involving largely the left cerebellar hemisphere, presenting with subarachnoid hemorrhage (SAH). (a) Left AP vertebral angiogram (early and late phases), showing the AVM supplied by branches of the posterior inferior (three arrows), anterior inferior (arrow head), and superior cerebellar (small arrow) arteries. The lateral pontine arteries (bidirectional arrow) are very dilated and probably also involved. Wall irregularities and several aneurysms, intranidal and also on the feeding branches, are recognizable. (b) Selective study of the posterior inferior cerebellar artery (PICA) preceding embolization showing better the multiple aneurysms

Fig. 12.12
Laterotemporal occipital AVM in a child, presenting with epileptic seizures. (a) Carotid angiogram, AP view. The AVM consists mainly of a direct fistula (arrow) between the gyrus angularis artery and the venous sector, characterized by a venous pouch directly communicating with the adjacent dilated vein. A typical plexiform nidus is not definitively recognizable. (b) Late phase, showing the cortical and deep drainage. The latter occurs through a dilated lateral atrial vein (arrow) continuing into the distal basal vein (BV). (c) Super-selective study showing more clearly the fistulous shunt and deep venous drainage. (d) Carotid angiogram, lateral view, early and late phase, showing the feeding arteries and drainage involving the lateral atrial vein (arrow). (e) Carotid angiogram, lateral view, after occlusion of the fistula with acrylic glue; a minimal network corresponding to the persistent nidus is still recognizable. This was treated later with radiotherapy

Fig. 12.13
Older patient presenting with severe SAH involving predominantly the right cerebellopontine angle. Vertebral angiogram (a) showing a small AVM in the cerebellopontine angle (arrowheads) supplied by a double superior cerebellar artery (large arrow) and dilated lateral pontine artery (small arrow). With the latter, an aneurysm, probably flow dependent, is recognizable. This was thought to be responsible for the SAH and was acutely occluded with coils (b), together with the parent artery. The comatose patient recovered well

Fig. 12.14
Older patient presenting with severe SAH involving predominantly the left cerebellopontine angle. (a) Vertebral angiogram (oblique view) showing the AVM supplied by branches of the superior cerebellar artery (large arrow). A lateral pontine artery (arrowhead) seems also to be involved. There is a further supply from the anterior inferior cerebellar artery (AICA, arrows). On its course, a flow-dependent aneurysm is recognizable. (b) Later phase, showing the drainage in the superior petrosal sinus. (c) Selective study of AICA preceding the occlusion of the aneurysm and distal AICA with coils. The patient recovered. The AVM was later treated with surgery

Fig. 12.15
Older patient presenting with SAH. A complete angiographic study showed a petrotentorial dural arteriovenous fistula (DAVF) on the right. (a) Right internal carotid angiogram, lateral view, showing the typical feature of the fistula supplied by cavernous branches of ICA. (b) Vertebral angiogram, AP view, disclosing, on the right, a well-developed AICA, partially supplying the DAVF (arrows) through its rostro-lateral branch (arrow). An aneurysm, probably flow dependent, is recognizable on the supplying artery


Fig. 12.16




(a, b) Severe SAH in an older patient. (a) Small cerebellar AVM (arrowheads) supplied by distal branches of the PICA was visible on the vertebral angiogram. A flow-dependent aneurysm (arrow) is recognizable on the supratonsillar segment of the PICA. This was occluded with coils (b). The patient remained comatose and died. (c–g) Another example of an old patient presenting with severe SAH involving predominantly the posterior fossa. The angiographic study revealed an AVM of the cerebellar vermis and partially of the right cerebellar hemisphere supplied by distal branches of the cerebellar arteries. Aneurysms, probably “flow dependent,” were recognizable on the course of the AVM feedings arteries. These were thought to be responsible of the SAH and treated acutely. The AVM was operated on later. (c, d) Right VA. The PICA is replaced by a well-developed AICA supplying the AVM. On one branch an aneurysm (arrow head) better visible on the selective study is recognizable. (e) Control angiogram post occlusion of the aneurysm with Onyx. (f, g) Left VA angiogram. Similar several aneurysms (arrow head and triple arrows) are visible on the supratonsillar and vermis branches of the large PICA. These are better demonstrated on the selective study, preceding treatment with Onyx. Normal posterior meningeal artery (arrow)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


