(1)
Department of Neuroscience, University of Turin Ospedale Molinette, Turin, Italy
Abstract
The first case of spontaneous dissection of the internal carotid artery (ICA) was reported in 1954 by Jentzer. Since then, other cases have been described (Bostrom and Liliequist 1967; Ehrenfeld and Wylie 1976; Fisher et al. 1978; Mokri et al. 1979; Anderson et al. 1980; Friedmann et al. 1980; Bradac et al. 1981a, b), and these have helped widen our understanding of the clinical and angiographic aspects of this disease. Spontaneous dissection is today a well-recognized pathology that is responsible for stroke in many cases. Its incidence is reported to be three to four cases per 100,000 persons yearly (Schievink 2001; Menon and Norris 2008; Redekop 2008). Young and middle-aged patients are predominantly affected.
16.1 Introduction
The first case of spontaneous dissection of the internal carotid artery (ICA) was reported in 1954 by Jentzer. Since then, other cases have been described (Bostrom and Liliequist 1967; Ehrenfeld and Wylie 1976; Fisher et al. 1978; Mokri et al. 1979; Anderson et al. 1980; Friedmann et al. 1980; Bradac et al. 1981a), and these have helped widen our understanding of the clinical and angiographic aspects of this disease. Spontaneous dissection is today a well-recognized pathology that is responsible for stroke in many cases. Its incidence is reported to be three to four cases per 100,000 persons yearly (Schievink 2001; Menon and Norris 2008; Redekop 2008). Young and middle-aged patients are predominantly affected.
16.2 Pathology and Pathogenesis
From a pathological point of view, the lesion is characterized by a subintimal hemorrhage, leading to stenosis or occlusion of the artery. The hemorrhage can involve the outer media or the sub-adventitial layer, resulting in the formation of pseudoaneurysms. Dissection occurs because of a primary intimal tear, allowing the blood to pass into the arterial wall. Primary intramural hemorrhage can also occur through rupture of the vasa vasorum.
The pathogenesis of dissection can be traumatic, but in the spontaneous form, the pathogenesis is not completely clear. Structural changes of the arterial wall, associated with mechanical factors, are probably involved. Indeed, it is well known that dissection occurs frequently in patients with fibromuscular dysplasia (FMD), Ehlers–Danlos syndrome, Marfan syndrome, and lupus erythematosus (Anderson et al. 1980; Mitsias and Levine 1994; Schievink et al. 1994a; North et al. 1995).
Association with cystic medial necrosis has also been demonstrated in some cases (Schievink et al. 1994a). Such cases are more frequent in patients with migraine and in women using oral contraceptives (Mokri et al. 1986; D’Anglejan-Chatillon et al. 1989). A family history has also been reported (Schievink and Mokri 1995). Trauma, even minor, and neck manipulation (Hufnagel et al. 1999; Nadgir et al. 2003) are present in the clinical history of some patients. In this context, the association of trauma, even minor, and raised blood alcohol as a cause of dissection, particularly in the vertebrobasilar sector, have been emphasized (Hiraiwa et al. 2005). Some authors (Konrad et al. 2003; Vila et al. 2003) have reported in cases of dissection a deficiency of alpha 1-antitrypsin. This is a proteinase that has a protective action on collagen and elastin—important components of the connective tissue of the arterial wall. The same deficiency has been identified in cases of FMD, which shows a high tendency for dissection (Schievink et al. 1998).
16.3 Location
Extracranial ICA: 2–3 cm distal to the bifurcation is the most frequent site of dissection. This commonly ends abruptly, with rare exceptions, where the artery enters the base of the skull. The second-most frequent location is the extracranial vertebral artery. Any part of the vertebral artery (VA) can be involved, but the lesions are most frequent in the distal segment of the artery, at C1–C2, and the atlas loop level because this segment is more subject to mechanical trauma (Chiras et al. 1985; Mokri et al. 1988; Shin et al. 2000; Schievink 2001). More than one vessel is involved in about 25 % of cases (Schievink 2001).
Intracranial dissection is less frequent. All ages can be involved, but there is a predominance of young patients. The vertebrobasilar sector is more frequently affected. Dissection usually involves the first intracranial segment of the VA, sometimes as an extension of extracranial dissection. Both VAs can be involved. Dissection of the basilar artery is less common; it can be primary or a secondary extension of dissection of the VA (Alexander et al. 1979; Shimoji et al. 1984; Berger and Wilson 1984; Friedman and Drake 1984; Caplan et al. 1988a; Pozzati et al. 1995; Shin et al. 2000; Lacour et al. 2000; Manabe et al. 2000; Kurata et al. 2001; Sugiu et al. 2005; Ramgren et al. 2005; Lee et al. 2006a; Zhao et al. 2007). Dissection is also frequently the cause of aneurysms of cerebellar arteries, more commonly of the posterior inferior cerebellar artery (Ramgren et al. 2005; Bradac and Bergui 2004; Mitsos et al. 2008) as well as the posterior cerebral artery (Lazinski et al. 2000; Roh et al. 2008) (see also Sect. 11.6.8). Dissection in the anterior circulation is rarer; it can be an extension of dissection of the ICA toward the intracranial sector. Intracranially, primary dissection typically involves the terminal ICA, the proximal segments of MCA and ACA, more rarely the distal branches (Ramsey and Mosquera 1948; Kunze and Schiefer 1971; Hochberg et al. 1975; Sasaki et al. 1991; Massoud et al. 1992; Bassetti et al. 1994; Nakatomi et al. 1997; Mizutani 1998; Ohkuma et al. 2003; Lv et al. 2009; Küker et al. 2012). Dissection involving PcomA can also occur (see Sect. 11.6.4).
16.4 Morphological Diagnostic Appearance
In the extracranial sector, occlusion with a typical tapered appearance is frequent as well as an irregular stenosis, which extends for various lengths. Demonstration of a double lumen or an intimal flap can be pathognomonic, but it is rare. Pseudosaccular aneurysm can also occur (Figs. 16.1, 16.2, 16.3, 16.4, 16.5, 16.6, and 17.4c).
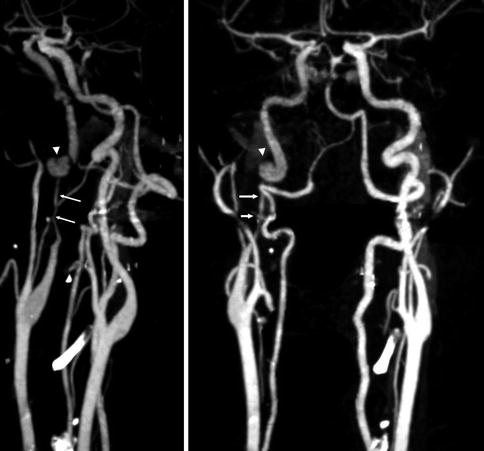
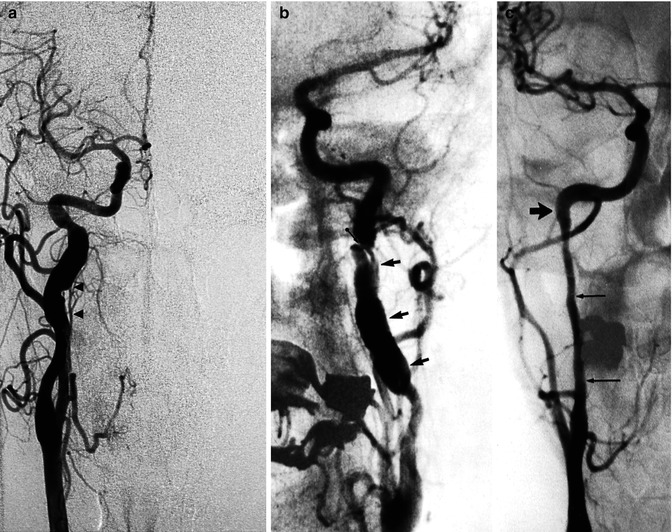
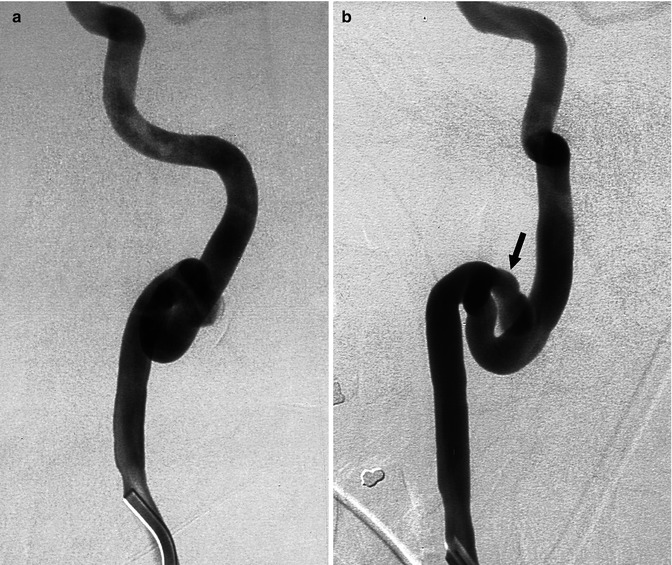
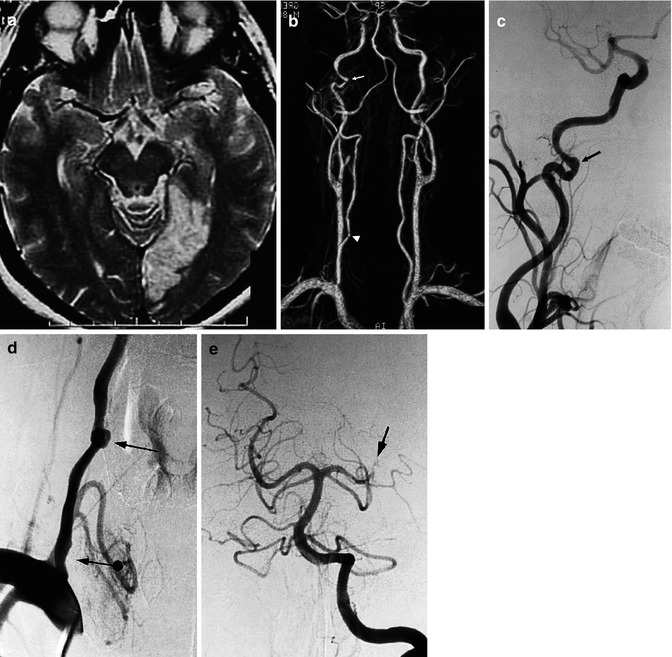
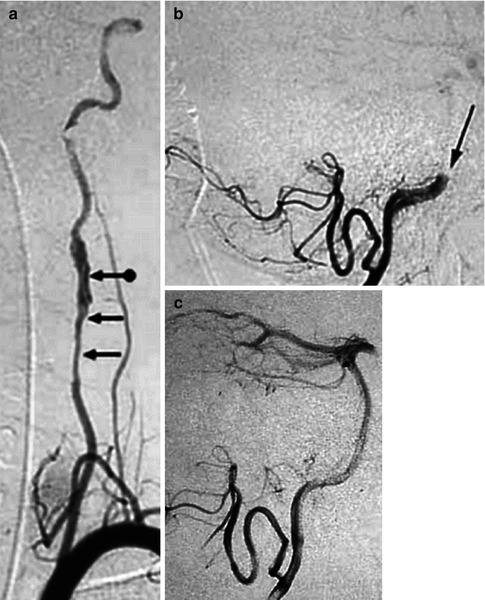
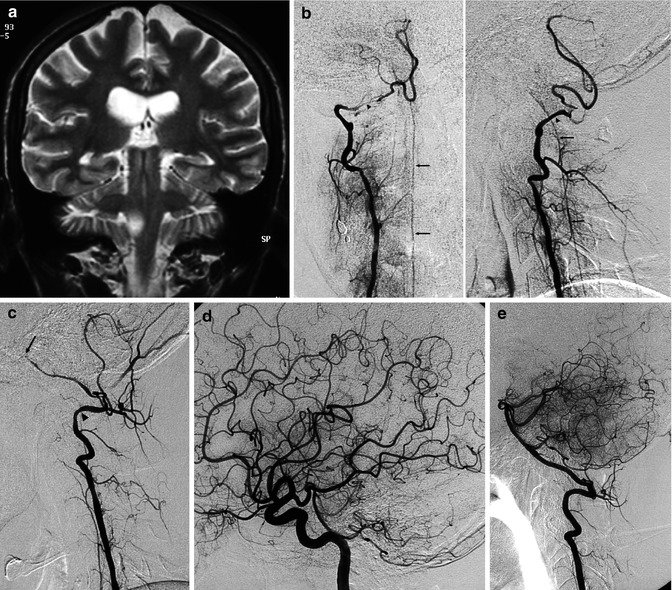

Fig. 16.1
CT angiography. Dissection of the right ICA characterized by a severe stenosis 2-cm distal to the bifurcation (arrows). A pseudoaneurysm is also present (arrow head). On the CT study there was a small infarct in the territory of the MCA probably embolic

Fig. 16.2
Example of spontaneous dissection of an extracranial ICA. (a) Irregular fusiform dilatation arresting at the skull base. Note the ascending pharyngeal artery (APhA; arrowheads) running close to the ICA. (b) Irregular fusiform dilatation of the ICA (arrows). A flap is recognizable (arrow with dot). (c) Very long stenosis arresting at the base of the skull

Fig. 16.3
Middle-aged patient presenting with acute cervicalgia. The complete angiographic study showed tortuosity of both ICAs. The right carotid angiogram (two oblique views). The right carotid angiogram, two oblique views (a,b) disclosed a prominent coiling associated with a probably large dissecting aneurysm (arrow). Conservative medical treatment was performed. Three months later, a control angiogram showed the unchanged aneurysm, which was occluded with coils

Fig. 16.4
Patient admitted with acute ischemia in the vascular territory of the left posterior cerebral artery, demonstrated on MRI. (a) MRI angiography, (b) showing irregularity of the right internal ICA (arrow), and a doubtful finding on the right vertebral artery (arrowhead). The angiogram of the right ICA (c) confirmed the changes to the artery, probably due to dissection (arrow). The right vertebral angiogram (d) showed mild stenosis of the vertebral artery at its origin (arrow with dot) and a rounded pseudoaneurysm, probably dissecting, at the level of C6 (arrow). This was probably the cause of the embolic occlusion (e) of the left posterior cerebral artery (arrow)

Fig. 16.5
Spontaneous dissection of the left vertebral artery in a very young patient, with embolic occlusion of the middle basilar artery. Left vertebral angiogram (a) showing irregularity of the artery with stenosis (arrows) and dilatation. A double lumen is also recognizable (arrow with dot). Occlusion (b) of the basilar artery (arrow). Selective catheterization with a microcatheter of the basilar artery through the right vertebral artery, followed by pharmacological fibrinolysis with reopening (c) of the artery. Complete recovery of the patient

Fig. 16.6
Young patient with brainstem stroke due to dissection of both vertebral arteries (VA) with involvement also of the basilar artery (BA). (a) MRI, coronal view, and T2-weighted image showing a small lateral bulbar ischemia. (b) Angiogram of the right VA (AP and lateral view) showing segmental narrowing of the VA, especially at the extracranial–intracranial passage (arrowhead). The VA ends in the posterior inferior cerebellar artery (PICA). There is injection of the anterior spinal artery (ASA; arrows) through a radiculomedullary artery. (c) Angiogram of the left VA, showing similar segmental narrowing of the VA (arrowhead) and BA, which appears occluded in its middle segment (arrow). (d) On the carotid angiogram, there is a good collateral circulation through the circle of Willis, with a retrograde injection of the BA. Medical therapy was performed. The patient improved rapidly and was symptom-free 1 month later. (e) Normal control vertebral angiogram
Intracranial dissection appears as occlusion and irregular stenosis (Figs. 16.6, 16.7, and 16.11), pseudoaneurysm, or fusiform dilatation (Figs. 5.8, 5.9, 11.21, 11.22, 11.23, 11.24, 11.25, 16.8, 16.9a–f, 16.10, 16.12, and 16.13). Not infrequently, the arterial changes are minimal. Repeated examination days or weeks later can show more conspicuous alterations (Fig. 16.9a, b).
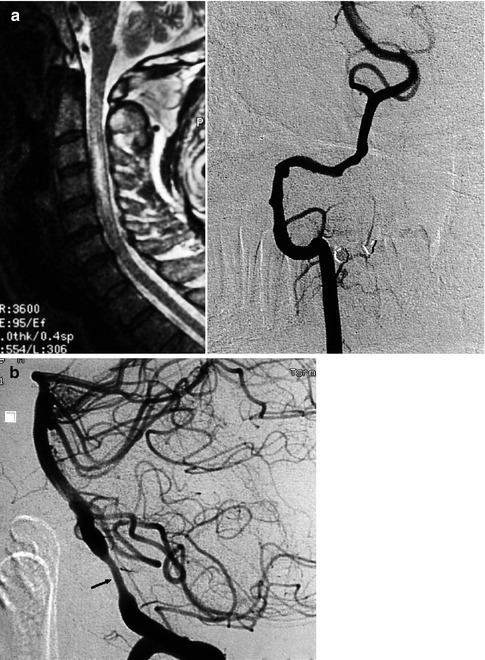
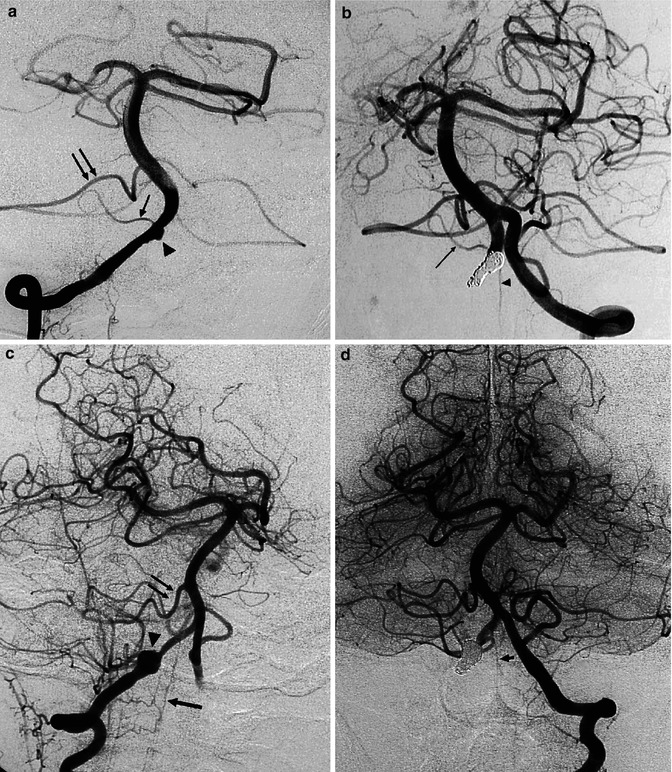
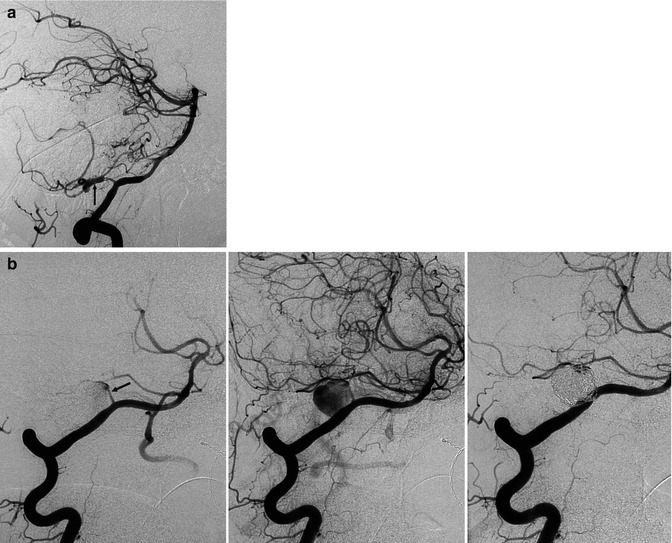
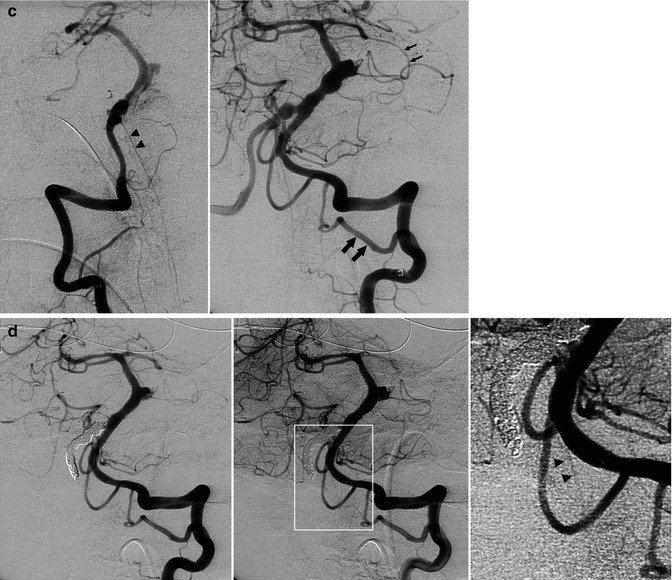
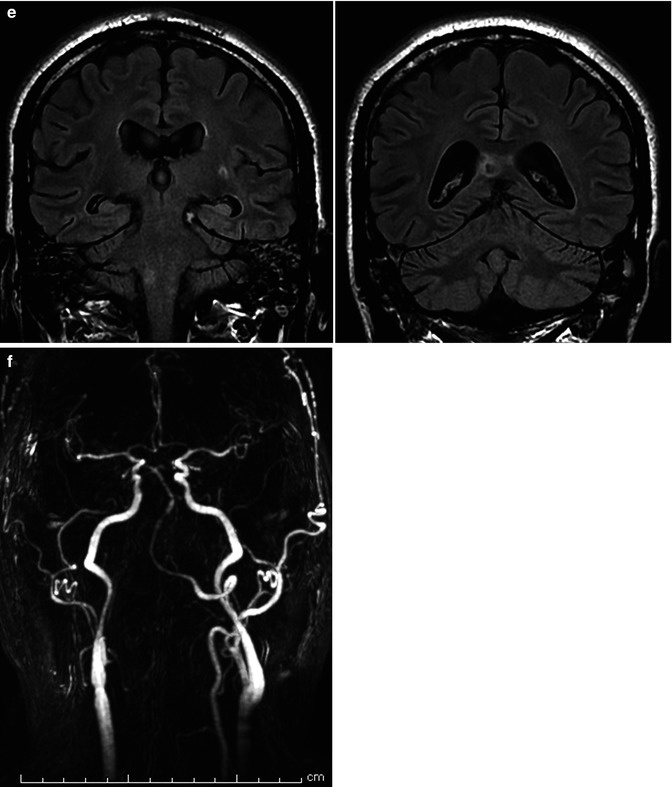
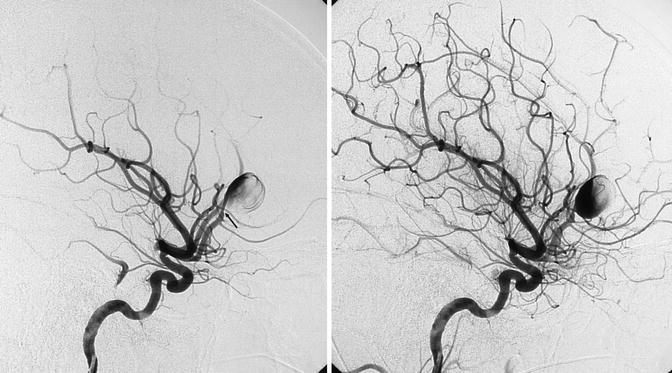

Fig. 16.7
(a) Spinal cord ischemia in a young patient due to dissection of the right vertebral artery. MRI T2-weighted image showing the hyperintensity lesion of the spinal cord. Right vertebral angiogram disclosing irregularity with a small pseudoaneurysmal dilatation of the distal extracranial and partially intracranial VA. The left VA was normal. The anterior spinal artery is not always visible on the vertebral angiogram. Its absence in this specific case, however, is probably due to occlusion. The patient recovered, and on the control angiogram 2 months later, the VA was normal. (b) Dissection of the left VA in another patient presenting with transient brainstem ischemia. Irregular stenosis is recognizable in the intracranial VA. Control angiogram 2 months later showed normalization of the lumen

Fig. 16.8
Dissecting aneurysm of the right VA, presenting with subarachnoid hemorrhage (SAH). (a) Right vertebral angiogram showing irregular small fusiform aneurysm (arrowhead) proximal to the origin of the small PICA (small arrow). A large anterior inferior cerebellar artery (AICA; arrows) supplies the vascular territory of the PICA. (b) Left vertebral angiogram after occlusion of the aneurysm with coils and of the right VA. The right PICA was preserved (arrow) as well as the anterior spinal artery (arrowhead), arising typically distal to the PICA. The patient recovered well. Another example of a dissecting aneurysm of the right vertebral artery presenting with SAH. (c) Right vertebral angiogram, oblique view. Round dilatation (arrowhead) of the VA. There is a large AICA (double arrow) supplying completely the vascular territory of the PICA. Anterior spinal artery (arrow). Well-developed PICA on the left. (d) Left vertebral angiogram after occlusion of the aneurysm and right VA. Anterior spinal artery (arrow)



Fig. 16.9
(a, b) Severe SAH in a relatively young patient. (a) Right vertebral angiogram showing fusiform dilatation, suggesting dissecting aneurysm (arrow) of the lateral bulbar segment of the PICA. The comatose patient recovered slowly. (b) The second angiogram 3 weeks later showed a tremendous enlargement of the aneurysm, now involving the PICA, also near its origin (arrow). Selective occlusion of the aneurysm with coils. Remark the filling of muscular branches of the vertebral artery arising from C2 level. (c–f) Middle-aged patient presenting with hemorrhage due to dissecting aneurysm involving the distal right VA and proximal and middle BA. (c) Right and left angiogram. On the left vertebral angiogram there is reflux in the right VA. A few anatomical variants are present. There is a very large left PICA (arrows) arising extracranially which supplies also the contralateral vascular territory. The left SCA (small arrows) supplies through a well-developed marginal branch also the AICA involved probably in the dissection. The ASA (arrows head) seems to be unilateral, arising from the right VA. On the Right VA angiogram (oblique view) there is confirmation of the origin of ASA (arrows head). Stents have already been positioned in the BA. (d) Control angiogram after endovascular treatment. Two flow-diverter stents (pipeline) were positioned in the BA, followed by occlusion with coils of the distal right VA. The unilateral right origin of the ASA was an unfavorable condition. However, the decision to occlude the VA was taken considering the presence of a large left VA and large left PICA. Perforators entering the medulla arising from VA, PICA, and ASA are end arteries. However, anastomoses, frequently rich, between these arteries are present on the surface of the medulla (Sect. 6.2.1). Furthermore, there is a possibility of collateral circulation toward the ASA from radiculomedullary artery from C3 to C4. The left vertebral angiogram (early and later phases with magnified detail) shows improvement of the BA and occlusion of the right VA. There is also a weak filling of the ASA possible through a retrograde minimal filling through the coils of the occluded right VA. The minimal flow defect in the proximal basilar was interpreted as a beginning thrombus which disappeared after selective injection of an antiplatelet agent. (e) The patient tolerated well the treatment. He had deglutition troubles which improved gradually. The MR performed 10 days later showed a small ischemic lesion in the right lateral medulla and in the right splenium. The latter probably embolic. Small old lesion in the white matter. (f) A month later, on the MR angiography a progressive morphological improvement of the basilar artery was recognizable

Fig. 16.10
Young patient presenting with a severe SAH hemorrhage with a parenchymatous component owing to rupture of an aneurysm, probably dissecting, of the proximal segment of the pericallosal artery. The arrow shows the small stenosis of the artery proximal to the aneurysm. In a later phase, progressive filling of the large aneurysm and distal pericallosal artery. The sac of the aneurysm was clipped, but a week later a second severe SAH occurred. A second angiographic study revealed that the aneurysm had enormously increased. It was occluded together with the pericallosal artery with coils
CT and MRI demonstrate ischemic lesions or, in cases of ruptured intracranial dissecting aneurysm, subarachnoid hemorrhage (SAH). Furthermore, MRI can show hyperintensity of the involved artery or dilatation of the vessel on T1-weighted sequences. Eccentric signal void (corresponding to the residual lumen) surrounded by a semilunar hyperintensity (corresponding to the mural hematoma) can be evident. This, however, may be visible only one or two days after the acute episode, first on T1-, then on T2-weighted sequences (Zuber et al. 1994; Levy et al. 1994; Hosoya et al. 1999; Provenzale 2009; Vertinsky et al. 2008).
MRI angiography and, particularly, CT angiography can replace angiography in many cases. But the latter, owing to its precise morphological selective demonstration of the arteries, still remains the gold standard and is to be used every time in young patients, if the diagnosis is not clear or if treatment (surgical or endovascular) is planned.
16.5 Clinical Relevance
Dissection most commonly occurs extracranially, and it is responsible for 2 % of all ischemic strokes and for about 20 % of all strokes in patients under 45 years of age (Bogousslavsky et al. 1987; Schievink 2001; Redekop 2008). Some dissections may be accompanied only by cervical and facial pain (Buyle et al. 2001), which are always present, and by Horner’s syndrome as a result of sympathetic fibers palsy in the ICA dissection. All these signs can elude an imprecise neurological examination.
In many cases, dissection leads to ischemia, commonly through emboli, involving the anterior or posterior circulation, depending on the artery involved. The spinal cord can also be infrequently involved in dissection of the VA (Weidauer et al. 1999; Crum et al. 2000).
An interesting aspect is palsy of the last four cranial nerves in dissection of the ICA in various combinations (Bradac et al. 1981a, 1989, 2000; Maitland et al. 1983; Hommel et al. 1984; Lieschke et al. 1988; Nusbaum et al. 1998; Waespe et al. 1988; Vargas et al. 1992; Schievink et al. 1993; Mokri et al. 1996; Sturzenegger et al. 1995). This can be due to compression of pseudoaneurysms, which, however, are not always present. It has also been supposed that a slight enlargement of the ICA could compress the ascending pharyngeal artery (APhA), which runs parallel and adjacent to the ICA (Bradac et al. 1989, 2000). Indeed, from previous studies, it is well established that the APhA supplies these nerves (Lasjaunias and Doyon 1978). The rare involvement of cranial nerves III, IV, and VI, supplied by branches of the inferolateral trunk (ILT), could be due to a slight extension of the dissection to the cavernous portion of the ICA. Hypoperfusion, or embolic involvement of the ILT, can also be taken into consideration (Bradac et al. 2000). Indeed, involvement of these nerves has been described in cases of ICA occlusion (Wilson et al. 1989; Kapoor et al. 1991).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


