Chapter 18
Vascular Laboratory
Venous Duplex Scanning
Luis R. León Jr.,, Nicos Labropoulos
Based on a chapter in the seventh edition by Luis R. Leon, Jr. and Nicos Labropoulos
Duplex ultrasonography (DUS) is the best screening, perioperative, and follow-up tool available for the evaluation of vascular disease. In the assessment of venous disease, DUS is used to detect acute deep venous thrombosis (DVT) and venous reflux and to evaluate chronic venous obstruction. It is also a great method to provide differential diagnosis and thus improve management of the patient. The progress achieved by DUS during the last 20 years has led to its current central role in the diagnosis of venous disease.
Basic Principles
DUS is a sensitive and specific tool for the assessment of most venous diseases. However, like many other modalities, it is operator and equipment dependent. There are considerable variations and training levels among investigators who perform this test. Reliable information can be obtained from DUS only by experienced operators who have extensive knowledge of venous anatomy, physiology, and pathology. Technologists, not always certified or registered, perform these examinations in the United States. Technologists, vascular scientists, and physicians perform these tests in many other countries. It is also common for surgeons, angiologists, and phlebologists to perform their own testing. It would be ideal if the personnel performing the investigations undergo systematic training. Proof of continuing sonographic education, at least yearly, should be also required.
Of all outpatient DUS examinations for suspected acute lower extremity DVT, only 12% to 25% detect an abnormality; an increasing prevalence of abnormal findings is seen in patients with pertinent symptoms or thrombotic risk factors.1–6 Until recently, there was no systematic consensus on how DUS is best performed for chronic venous disease. The American College of Radiology published practice guidelines for the performance of peripheral venous DUS in 1993 (last revision in 2006). The clinical aspects of this document (indications, specifications of the examination and equipment) were developed collaboratively by the American College of Radiology and the American Institute of Ultrasound in Medicine. In 2006, a consensus document on DUS investigation of the veins in chronic venous disease of the lower limbs, released in two parts by the Union Internationale de Phlébologie,7,8 summarized the current standards for assessment of the lower extremity veins with DUS.
All maneuvers and techniques that will be used during the examination should be explained to the patient. Certain conditions regarding equipment and examination technique must be met for optimal results to be achieved. Enough lighting should be provided to enable a thorough evaluation of the lower limbs and to establish the distribution of varicosities. The lower extremities should be inspected for varicosities and scars from surgery to help predict the source of reflux and to facilitate the examination. The room should also be warm and comfortable to ensure that there is no vein spasm, which would hinder accurate determination of vein size. The importance of these seemingly trivial suggestions was pointed out by van Bemmelen and associates.9 By modifying room conditions at the time of DUS, they showed that vein diameters are significantly larger after warm-water submersion in a sitting position without a tourniquet than after other conditions.
Choosing the right transducer for a certain patient, illness, or vein is important. Axial resolution and tissue penetration are inversely related. A 4- to 7-MHz linear array transducer is optimal for assessing most veins, which normally lie between 1 and 3 cm below the skin. Deep veins, such as those in the abdomen or pelvis, or veins in obese or edematous patients are better assessed by using lower frequency curvilinear transducers for deeper penetration.
Low blood flow settings are used most often. The pulse repetition frequency (the number of pulses transmitted per second) is set at 1500 Hz or lower. When vein stenosis or arteriovenous fistula is suspected, pulse repetition frequency should be increased because blood flow velocity is considerably elevated. The imaging focus should be set at the far wall (in relation to the skin) to achieve better lateral resolution. The lumen of the vein should be set to appear dark in the absence of stasis and thrombosis. Time-gain compensation (increasing amplification of ultrasound echoes with depth to compensate for their progressive attenuation) is set according to the echogenicity and location of the examined tissues to improve imaging. The weaker the signal seen with increasing depth, the higher the gain that will be required. When velocity waveforms are obtained, gain should be set so that the background is dark to avoid overestimation. The insonation angle is often set at 0 degrees. However, because most veins run parallel to the skin, this angle has to be set to parallel the vein flow channel. An angle of insonation of 45 to 60 degrees between the transducer and the vein should be used to achieve the optimum Doppler waveform. This is done only when the velocity of reflux is estimated.
Thrombosis
Prompt and accurate diagnosis of DVT has the potential to prevent major morbidity. Clinical diagnosis of DVT is misleading in roughly 50% of cases because of its low sensitivity and specificity.10–13 As other methods were developed, ascending contrast-enhanced venography became the “gold standard.” DUS has replaced contrast-enhanced venography as the method of choice because of its high accuracy, portability, availability, noninvasive nature, and ability to perform a differential diagnosis.14–18
Venous obstruction can be caused by extrinsic and intrinsic conditions. Tumors, hematomas, cysts, aneurysms,19,20 and musculoskeletal structures can cause extrinsic vein compression. Such pathologic processes are not usually associated with signs and symptoms of chronic venous disease unless concomitant reflux or obstruction is present. Diagnosis of these conditions is important because it can alter management.
However, the most common cause of obstruction is venous thrombosis. DVT is a significant cause of morbidity and mortality in hospitalized patients. In the acute phase, pulmonary embolism and pulmonary hypertension can develop. Venous thromboembolism is the leading cause of preventable in-hospital mortality in the United States. On the other hand, postthrombotic syndrome describes the sequelae of chronic venous disease. Typical signs and symptoms are lower extremity pain, aching, cramps, tiredness, or heaviness; restless limbs; burning sensation or itching; chronic limb swelling; spider, reticular, or varicose veins; skin changes; atrophie blanche; lipodermatosclerosis; and ulceration.
DUS evaluation of the veins can determine the presence of anatomic obstruction with a sensitivity and specificity of more than 90%.21 Functional evaluation of obstruction cannot be achieved with DUS because it assesses a single vein segment at a time. Unlike the case of vein reflux, no adequate tests exist for the accurate measurement of functional obstruction.
Examination
For performance of the examination, the patient is placed in a reverse Trendelenburg position with the knee bent and in external rotation. The examination begins below the inguinal ligament at the common femoral vein (CFV) and the saphenofemoral junction (SFJ). The transducer is placed in a transverse orientation to the vein and compression is applied. The transducer is then turned longitudinally to evaluate for flow and augmentation. The veins are examined in 3- to 5-cm intervals. In a similar manner, all the deep veins of the extremity, including the femoral, deep femoral, popliteal, peroneal, soleal, gastrocnemius, and posterior tibial veins, are examined. It is important to bear in mind the possibility of anatomic variation in venous anatomy. For instance, duplications of the femoral vein were seen in 26% and triplications in 1.2% of consecutive patients referred for abdominal or lower limb imaging. These figures were 37% and 2.6% for the popliteal vein, respectively.22 Unless local signs or symptoms are present, the anterior tibial veins are not routinely examined because of their low incidence of DVT.23 The saphenous vein trunks are then evaluated.
It is crucial to assess the iliac veins and the inferior vena cava (IVC) when disease is suspected at that level. In this case, however, flow is evaluated chiefly because compression can be difficult and uncomfortable. Asymmetry of flow velocity, waveform, and pattern at rest and during flow augmentation in the CFV indicates proximal obstruction. However, the absence of asymmetry cannot exclude obstruction. Accordingly, when iliocaval obstruction is suspected, the full extent of these veins must be imaged. The presence of stenosis, usually from extrinsic compression, is recognized by the mosaic color (which denotes post-stenotic turbulence), abnormal Doppler waveform at the stenotic area, slow flow, spontaneous contrast, and vein dilatation before the stenosis.24 The reduction in vein diameter can be measured by planimetry to compare the smallest lumen with the normal lumen and by the peak vein velocity ratio (post-stenotic/prestenotic). The four components that should be examined are visualization, compressibility, flow, and augmentation.
Signs and symptoms are present in both lower extremities in patients with bilateral vein obstruction or when the IVC is involved. The iliac veins and IVC may exhibit extrinsic compression from masses. Such compression can lead to signs and symptoms of chronic venous disease, and in affected patients, thrombosis is a common event. Veins are either totally or partially obstructed and completely or partially recanalized. Timely recanalization may help maintain valve competence. Otherwise, the valve leaflets are damaged or destroyed.
Deep Venous Thrombosis
It is important not only to diagnose DVT accurately but also to determine whether the thrombotic event is acute or chronic because of considerable differences in management. DUS allows such differentiation,10,25 but no reliable methods are currently available to calculate thrombus age precisely. Different methods have been tested, including triplex ultrasound,26 magnetic resonance imaging of the thrombus (signal changes over time),27 and DUS (quantification of thrombus echogenicity)—all with mixed results.28
Acute Thrombosis
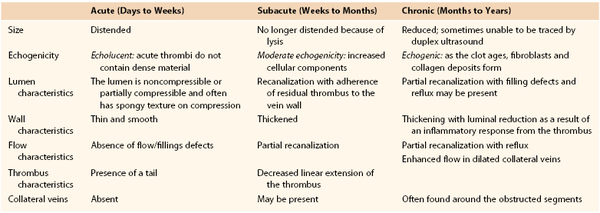
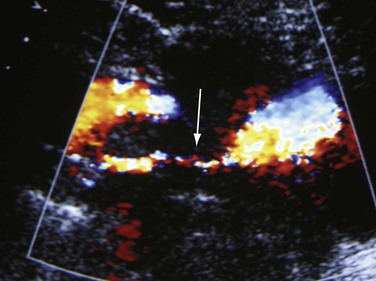
A fresh thrombus is mostly hypoechoic, homogeneous, partially compressible, seen in a dilated vein, and sometimes floating.10 Veins with acute thrombosis are echolucent and distended with smooth walls. Acute thrombus is spongy on compression, but it will still keep the walls of the vein from coapting. On color-flow examination, acute thrombus will have confluent flow channels (Fig. 18-1).
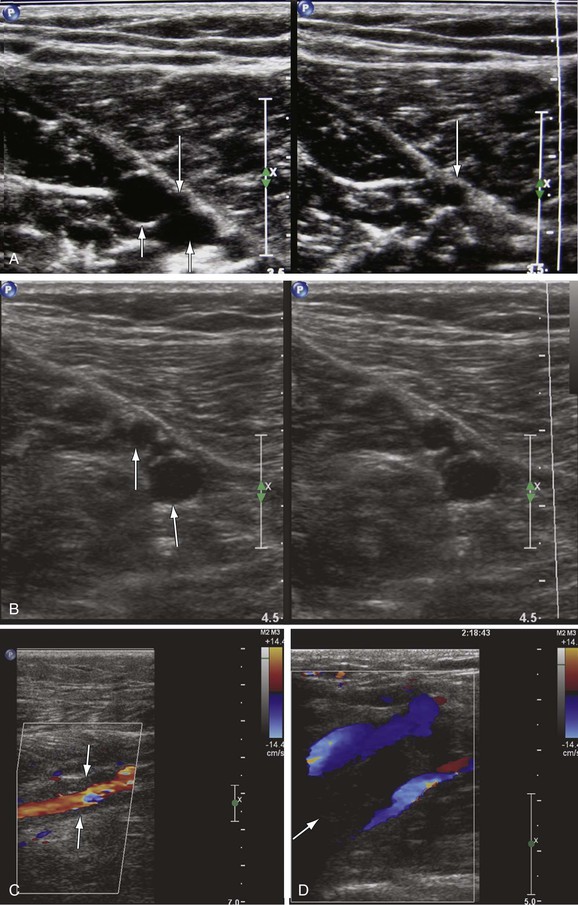
Figure 18-1 Diagnosis of acute deep venous thrombosis with duplex ultrasound. A, Cross-sectional view of the posterior tibial veins at the midcalf. Two veins (upward arrows) and the artery (downward arrow) in the center are seen below the soleus muscle (left panel). Both veins were collapsed normally with pressure applied by the transducer, so that only the artery is seen (right panel), indicating absence of thrombus within the veins. B, Cross-sectional view at the midcalf in a patient with acute calf pain. The posterior tibial veins (arrows) are distended and not compressible. C, Thrombosis of both posterior tibial veins in a high-risk asymptomatic patient. The artery is patent (red), and the accompanying veins (arrows) demonstrate absence of flow. D, Longitudinal view of the left common femoral vein from a patient with acute swelling and pain. There is a free-floating thrombus (arrows) surrounded by flowing blood at its proximal end.
Chronic Thrombosis
Chronic thrombosis is characterized by the presence of an organized, hyperechoic, heterogeneous, noncompressible thrombus firmly adherent to the vein wall on DUS.10 Chronic thrombus is firm. Chronic thrombi are echogenic and contracted with thick and irregular walls (Fig. 18-2). Chronic thrombus may have multiple channels or collateralization. Intraluminal webs and wall thickening, with or without reflux, indicate previous thrombosis in the absence of a visible thrombus. The presence of dilated collateral veins is a sign of obstruction, but their absence cannot exclude it. It is also possible for the veins to be fully recanalized without any evidence of anatomic obstruction. However, the thickening and increased stiffness of the vein wall can cause functional obstruction. Criteria for differentiation of acute from subacute and chronic DVT are depicted in Table 18-1.
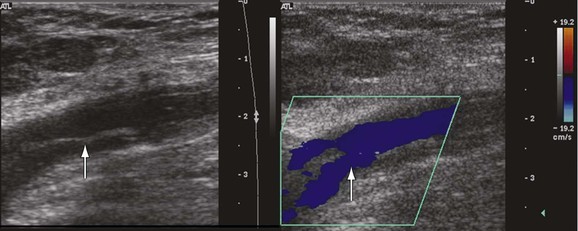
Figure 18-2 Chronic luminal changes from a past thrombosis of the common femoral vein in a patient with chronic swelling that became worse over time. Bright echoes in the lumen (arrow, left panel) as well as irregular flow channels (arrow, right panel) are seen.
Recurrent Thrombosis
Recurrent thrombosis is defined as a repeated thrombotic event. It may occur in the contralateral or ipsilateral limb. New thrombus may be formed in new vein segments that were not involved in the previous episode or in segments that previously had thrombosis (Fig. 18-3). After monitoring 738 patients for 3.7 to 8.8 years, Hansson and colleagues found a 5-year incidence of recurrent DVT of 21% after the first episode of DVT and 27% after a second episode.29 The cumulative incidence of pulmonary embolism was 2.6%. Proximal DVT, cancer, and a previous history of venous thromboembolism were identified as risk factors for recurrence. Patients with postoperative DVT and a longer duration of anticoagulation were at a lower risk for the development of DVT.29 Others have confirmed that patients with iliofemoral DVT have a twofold higher risk for development of recurrent DVT than do patients without iliofemoral involvement.30 Three DUS criteria can be used to diagnose recurrent DVT: extension of the thrombus more than 9 cm,31 noncompressibility of a vein segment that had previously been compressible or had previously recanalized, and increase in thrombus thickness by 4 mm.32 Patients with a single DVT episode must be monitored closely for recurrence. Patients who are at lower risk should undergo repeated DUS at 6 months before cessation of anticoagulation. Those at higher risk for recurrence may require a longer duration of anticoagulation, and a second DUS examination should be performed at a 1-year interval. Further studies are needed to determine the optimal duration of anticoagulation for patients who are at higher risk for recurrent DVT. DVT often recurs in new vein segments, and on such occasions the diagnosis is straightforward. When it occurs in previously thrombosed segments, the criteria of thrombus extension and thrombus thickness are used. In these instances, accurate recording of the baseline event and a subsequent follow-up scan are important for the diagnosis of recurrent events.
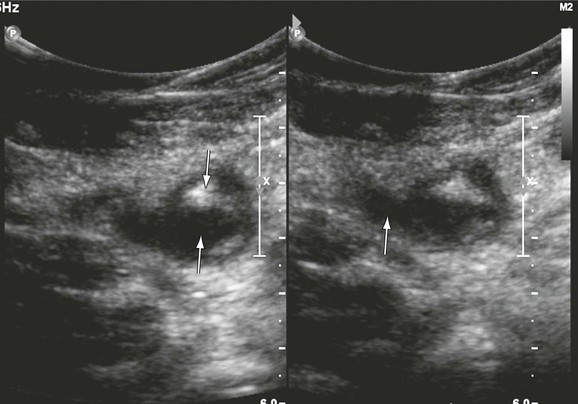
Figure 18-3 Recurrent thrombosis in the common femoral vein in a patient with limb swelling and chest pain. The patient previously had a femoropopliteal thrombosis documented in our hospital. The vein contains both echogenic (downward arrow) and echolucent (upward arrow) material (left panel). It is dilated (significantly larger than the common femoral artery, arrow, right panel) and noncompressible.
Central Vein Stenosis
Criteria have been developed to detect central vein stenosis by DUS in patients with signs and symptoms of venous outflow obstruction.24 Patients with limb swelling or pain (or both) and vein stenosis (41 stenoses in 37 patients) detected by DUS subsequently underwent contrast-enhanced venography with measurement of the pressure gradient. The gradient had to be 3 mm Hg or greater for patients to be included in this analysis. The best sonographic criterion for detecting significant vein stenosis was found to be a V2/V1 ratio of greater than 2.5 (with only two false-positive and false-negative test results reported) (Fig. 18-4). However, this was only a pilot study, and further studies need to be done to evaluate the best DUS parameters for hemodynamically or clinically significant vein stenosis. Planimetric evaluation by calculating directly the percentage diameter stenosis, vein dilation before stenosis, mosaic color from the turbulent flow at the exit of the stenosis, reduced flow augmentation, and presence of collateral veins are indicative of the diagnosis. The percentage stenosis may vary throughout the cardiac and respiratory cycles as the vein diameter can change. This is important in evaluating iliac vein compression by the iliac arteries. In patients with nutcracker syndrome (renal vein compression by superior mesenteric artery), we have used a vein velocity ratio of 4 to 5 as an indicator of significant stenosis, often combined with B-mode–measured diameter reduction.
Superficial Vein Thrombosis
Also known as superficial thrombophlebitis, superficial vein thrombosis is most often found in the extremity veins, but it can occur in many other locations. Although its diagnosis was based solely on clinical assessment, use of DUS has enabled accurate detection of its extent and progress (Fig. 18-5).
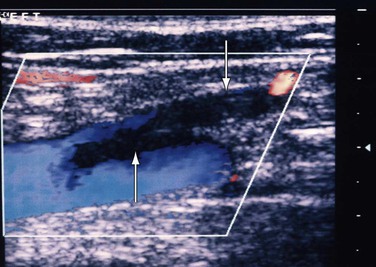
Figure 18-5 Extension of thrombosis from the thigh segment of the great saphenous vein (downward arrow) to the common femoral vein (upward arrow). The patient had pain in the lower part of the thigh, and within a week the thrombus extended proximally.
Thrombosis occurs most commonly in the saphenous veins and their tributaries, followed in frequency by the cephalic and basilic veins. Propagation of thrombus can occur in a contiguous or noncontiguous fashion. Contiguous extension of the thrombotic process from the superficial to the deep veins can occur in three ways, most commonly from the great saphenous vein (GSV) to the femoral vein. Less often, the thrombus extends from the small saphenous vein (SSV) to the popliteal vein. Extension through perforator veins to deep veins can also occur. In addition, it is possible that thrombosis can extend from deep veins to superficial ones, but this has not been documented. Most patients with superficial thrombophlebitis should have their deep veins evaluated even if the lowest prevalence of DVT ever reported (5.6%) is accepted.33 The diagnosis of superficial thrombophlebitis can be made on the basis of clinical or DUS data. Superficial thrombophlebitis is often easy to diagnose because of its superficial location, but the correlation between clinical examination and surgical findings is poor. Clinical examination does not reveal the true extent of superficial thrombophlebitis; surgical exploration often shows extension of the thrombotic process 5 to 10 cm higher than the level that was clinically diagnosed. DUS is recommended for confirmation of the diagnosis, for estimation of the extent of thrombosis, and for follow-up.33
Unusual Sites of Venous Thrombosis
The precise anatomic distribution of venous thrombosis in “unusual locations” has rarely been addressed. Among 15,850 DUS examinations performed in a 10-year period to rule out DVT, our group studied patients with DVT in all thigh veins except the femoral vein.34 The deep femoral, femoropopliteal, lateral thigh, sciatic, and muscular thigh veins were examined. We identified 14 cases (7 men, 0.54% of patients with DVT, and 0.088% of the entire population) involving thrombosis in unusual locations (Fig. 18-6). Ten cases involved the left lower extremity and 4 the right. The veins involved were the deep femoral (8); the femoropopliteal (2), which connects the popliteal with the deep femoral vein; and the deep external pudendal (1). Of the three patients with venous malformation, one had involvement of the muscular thigh veins, and in two patients the lateral thigh vein and the sciatic vein were thrombosed. Propagation of thrombi with extension to the CFV was seen in 4 of the 14 patients: 2 from the deep femoral vein, 1 from the femoropopliteal vein, and 1 from the deep external pudendal vein. Pulmonary embolism occurred in two patients, one of which was fatal. At final follow-up, recurrent DVT developed in two patients, and nine had signs and symptoms of chronic venous disease.34
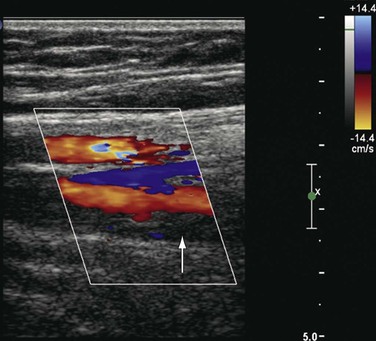
Figure 18-6 Acute thrombosis in the deep femoral vein in a high-risk asymptomatic patient. There is color filling (top to bottom) in the femoral artery and vein and in the deep femoral artery but not in the deep femoral vein (arrow).
The incidence of DVT of the thigh veins studied was extremely low. The signs and symptoms in our patients, both initially and at follow-up, spanned the whole spectrum of the natural history of DVT as reported in the literature for the more usual sites of thrombosis. Cases of thrombus propagation, pulmonary embolism, recurrent DVT, and postthrombotic syndrome were seen. The association of these veins with all the complications of thromboembolic disease suggests that routine imaging of these veins would be beneficial.34
Differential Diagnosis and Incidental Findings
DUS can be helpful not only when it yields a positive result for the diagnosis of a lower extremity ulcer, such as severe reflux of the posterior accessory vein of the calf, but also when the results show a completely normal venous system by extending the evaluation to rule out other causes. A multi-institutional study of 799 limbs with leg ulcers at a vein clinic reported 17 patients with 21 ulcers of nonvenous, nonarterial etiology.35 Fourteen of these patients had completely normal arterial and venous findings on DUS. The normal DUS results in these 14 patients prompted a search for alternative causes. Chronic inflammation, vasculitis, Kaposi’s sarcoma, chronic inflammation, basal cell carcinoma, pyoderma gangrenosum, hydroxyurea administration, and sickle cell disease were identified as the most prevalent causes for most of those ulcers.35 Venous and arterial DUS is recommended before any other test because of the overwhelming prevalence of venous and arterial disease in limbs with ulcers. In addition, many ulcers may have a mixed etiology, and venous and arterial testing can demonstrate the specific vascular disease that may contribute to ulcer formation.35
Several other conditions that mimic the symptoms and signs of venous disease can be diagnosed with DUS. Among them, Baker’s cysts, enlarged hematomas, lymph nodes, musculoskeletal injuries, aneurysms, and tumors may be identified. This is particularly important because some of these diagnoses may have a crucial impact on the therapy chosen (Fig. 18-7). For instance, when DUS is ordered to exclude DVT in a patient with the initial symptom of leg swelling and a hematoma or Baker’s cyst is found, anticoagulation becomes ill advised.36 Large ruptured Baker’s cysts can rarely compress the adjacent neurovascular bundle, leading to neurogenic symptoms.37 Patients with muscle rupture, hematoma, and ruptured Baker’s cyst are symptomatic and D-dimer levels are positive. Anticoagulation may be given inappropriately in these cases before objective testing, particularly if the patients present at hours when DUS may not be available.
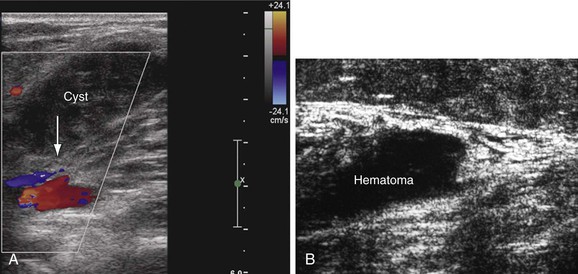
Figure 18-7 Incidental findings in patients who underwent ultrasound examination to rule out deep venous thrombosis. A, Hemorrhagic, ruptured popliteal cyst partially compressing the popliteal vein (arrow). An acute onset of pain and swelling developed the day before the examination. B, Rupture of the gastrocnemius muscle at its distal end near the origin of the Achilles tendon. The architecture of the muscle fibers is destroyed, and hematoma is seen over the area of the rupture.
Upper Extremity Veins
Upper extremity DVT has not been as extensively studied as lower extremity DVT. They have similar clinical findings: limb swelling or discoloration (or both) and pain and tenderness to palpation. However, these symptoms are nonspecific, and less than 50% of all symptomatic limbs will have imaging proof of DVT.
Compared with contrast-enhanced venography, the sensitivity and specificity of DUS have been reported to be 78% to 100% and 82% to 100%, respectively. However, except in highly specialized centers, contrast-enhanced venography remains the standard in more thorough evaluations of upper extremity DVT. Whereas false-positive results are rare, false-negative results may occur with small nonobstructive thrombi that cannot be directly assessed with compression techniques because of bones overlying centrally situated veins, including the medial subclavian vein segment, the brachiocephalic vein, and their confluence with the superior vena cava. Furthermore, differentiation between a normal vein and large collaterals in patients with chronic venous thrombosis may be difficult. Therefore, contrast-enhanced venography or magnetic resonance venography (MRV) is used in select cases in which DUS findings are equivocal or when clinical suspicion for upper extremity DVT is high despite negative DUS results.
Technique and Normal Anatomy
The patient should be placed in the supine position with the arm comfortably extended to approximately 60 degrees from the chest. Hyperextension should be avoided because it may inhibit normal flow and affect waveform shape and amplitude. Routine DUS examination includes assessment of the internal jugular vein, the brachiocephalic vein, the confluence of the cephalic and subclavian veins, the subclavian vein, the axillary vein, the brachial vein, the basilic vein, and the veins of the forearm. Evaluation of the brachiocephalic veins and superior vena cava requires a supraclavicular or suprasternal approach, and a sector or phased array transducer with a small footprint may be needed to facilitate imaging in areas surrounded by bone. The jugular vein is then scanned with the patient’s head rotated away from the side of examination. This testing is best performed with 5- to 7-MHz linear array transducers. Curved array transducers may be useful in the proximal axillary vein in larger patients to achieve better penetration and to obtain a larger field of view so that longer deep vein segments can be visualized. The superior vena cava and brachiocephalic veins are best imaged with small footprint transducers superior to the clavicle and through the sternal notch. The subclavian vein is imaged with the transducer placed above and below the clavicle. When it is viewed from beneath the clavicle, the vein lies superficial and caudal to the adjacent artery. The proximal axillary vein is seen below the clavicle under the pectoralis minor. The rest of the vein is imaged through the axillary area. Next, by placement of the transducer over the midportion of the inner aspect of the upper part of the arm in a transverse orientation, the brachial artery can be seen in the midsection of the upper part of the arm and used as a landmark. The paired brachial veins are closely applied to the artery.38 It is important to be aware of the multitude of anatomic variations of the arterial system in the upper extremity because these paired veins will change accordingly.39
The basilic vein lies more superficial. Although it is technically a superficial vein, the basilic vein is often larger than the brachial veins and may have a considerable thrombus burden. It unites the brachial vein at the midarm region or more proximally. Once the arm veins are interrogated, they can be followed centrally into the axillary vein. Waveforms should be obtained along the longitudinal vein axis at a Doppler angle of 60 degrees or less.
Knowledge of the normal spectral waveform in the upper extremity veins is essential for examination of these veins. The spectral Doppler signals are characterized by two phasic variations in amplitude. Cardiac pulsatility is manifested as a choppy and sometimes biphasic flow pattern that is more prominent in the central veins. Peak forward flow occurs during mid-diastole, whereas flow slows or reverses as the tricuspid valve closes. Respiratory variation may also be pronounced in the upper extremity veins, with increased flow during inspiration and decreased flow during expiration. With end-inspiration or end-expiration, little flow may be seen in healthy subjects. This should not be mistaken for blockage or an anechoic thrombus. Bilateral DUS is recommended for detecting waveform asymmetry, which may indicate more central obstruction that is difficult to directly visualize. As in the case of lower extremity examination, noncompressibility is the most sensitive and specific sign of venous thrombosis. In this area, compression is preferably done in the transverse plane because the transducer may slide off the vessel in the longitudinal axis and potentially result in false-negative results. Color Doppler imaging becomes useful when overlying bones prevent direct compression (Fig. 18-8).38
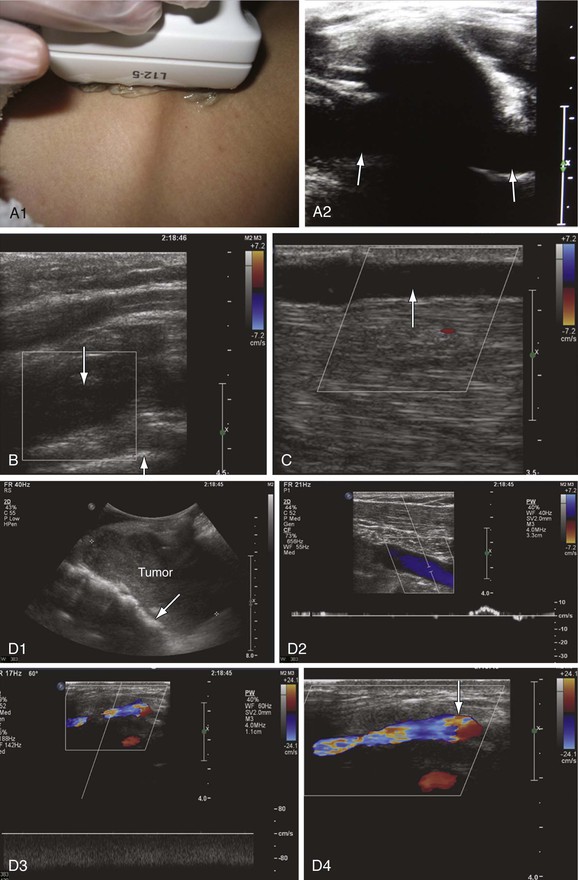
Figure 18-8 Upper extremity venous examination. A, Imaging of the subclavian vein through the clavicle (A1). This is an area where imaging has been difficult. However, use of the clavicle and pleura as landmarks makes imaging of this segment easier (A2). The subclavian vein is seen on both sides of the clavicle (arrows). B, Acute thrombosis of the proximal subclavian vein in a patient with acute swelling and pain in the left upper extremity. The vein is distended with absence of flow (down arrow). The white line crossing the right bottom corner of the color box is the pleura (up arrow). C, Spontaneous thrombosis of the cephalic vein in a patient with pain along the length of the vein for at least 2 days before the examination. The cephalic vein is dilated, with echolucent material in the lumen and absence of flow (arrow). D, Compression of the proximal left subclavian vein (arrow) by a tumor (D1). There is very low flow with poor augmentation in the axillary vein distal to the site of compression (D2). The venous flow is shifted from left to right through the jugular arch vein, which is dilated with high nonphasic flow (D3). The jugular arch vein diameter measures 5.6 mm in its largest segment (arrow) (D4). This vein is usually very small and is seen only in cases of proximal obstruction.
Diagnostic Criteria
Criteria for diagnosis of acute DVT in the veins of the upper extremity are identical to those described for their lower extremity counterparts, with some peculiarities. The difficulty of directly visualizing central vein thrombus makes examination of the upper extremities more reliant on indirect findings. Collaterals are very often visualized as prominent venous structures in the soft tissues surrounding the thrombosed main vein. Spectral analysis may also be useful in the evaluation of central thromboses in which the thrombus may be difficult to identify. Conversion of the normal biphasic pattern to a nonpulsatile signal similar to portal venous flow is strongly suggestive of central venous impairment, such as thrombosis, stenosis, or extrinsic compression by an adjacent mass (see Fig. 18-8). In cases in which a diagnosis of central thrombosis is considered on the basis of dampening of the spectral waveform, comparison should be made with the opposite side to confirm that the pattern is present only on the symptomatic side. Absent or reduced cardiac pulsatility was found to be a sensitive parameter. Respiratory phasicity is also a helpful finding. It is often asymmetrical in patients with unilateral venous occlusion. However, in cases of bilateral subclavian vein or superior vena cava occlusion, subtle dampening of pulsatility or phasicity may be difficult to appreciate. In situations with equivocal DUS results, especially when the clinical signs are highly suggestive of DVT, MRV or contrast-enhanced venography is advised for further evaluation.38
Not uncommonly, the only manifestation of chronic venous thrombosis is an inability to show a specific vessel in the expected anatomic location because the vein is simply collapsed and fibrosed. Collateral veins may become so prominent in patients with chronic disease that they may be mistaken for the main vein, which is essentially absent. In patients thought to have chronic thrombosis, any deviation from the expected normal anatomy or flow direction should be viewed with suspicion. Identification of the vein and its adjacent artery is also an important clue to differentiating main veins from enlarged collaterals. In addition, the loss of respiratory phasicity and in particular cardiac pulsatility by spectral analysis may be the only positive finding in patients with central venous obstruction who have otherwise normal findings. A caveat of using this criterion is that sometimes these collateral vessels are large and extensive enough to provide adequate drainage, and pulsatility and respiratory variations may be preserved.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree