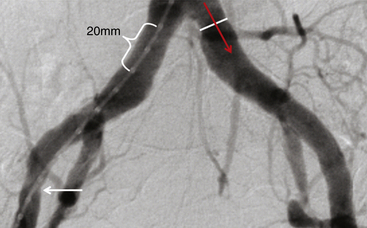
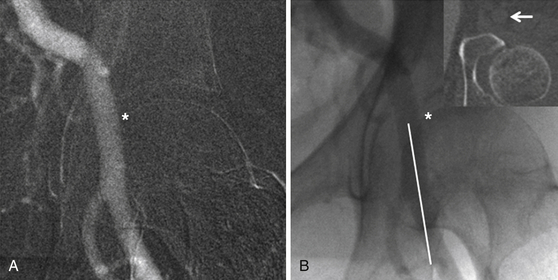
Chapter 3 Most of the renewed interest in arterial access has coincided with the advent of transcatheter aortic valve replacement (TAVR). Similar to the devices developed for endovascular aneurysm repair (EVAR), 16F to 24F sheaths are required to deliver transcatheter heart valves (THVs) from a retrograde transfemoral approach. Early in the experience of TAVR, a surgical cutdown with arteriotomy was often used for femoral arterial access and closure given the significant morbidity and mortality associated with vascular complications.1 Smaller sheath sizes, advanced interventional techniques, and suture-mediated percutaneous closure devices have now made a completely percutaneous approach to TAVR and other structural heart disease procedures possible. This minimally invasive technique holds the most promise for the future of the procedure and, if done correctly, can dramatically decrease procedure time, postprocedure morbidity, and in some cases obviate the need for general anesthesia. Several steps are necessary to safely perform a completely percutaneous transfemoral TAVR: appropriate patient selection, proper procedural technique, and postprocedural management. Understanding which patients are candidates for a transfemoral approach to TAVR is the first step to a successful procedure. Large body mass index (BMI) or body surface area, high sheath–to–femoral artery ratio (SFAR), femoral artery calcification, peripheral arterial disease, and low operator experience have all been associated with high complication rates and poor outcome for a percutaneous transfemoral approach.2–5 Patient-specific factors like BMI and prior peripheral arterial disease are known at the time of a clinic visit, but imaging is crucial for evaluating the vessel-specific factors of size, calcification, and tortuosity. Imaging of the femoral and iliac vessels is performed by invasive angiography, contrast and noncontrast computed tomography (CT), magnetic resonance (MR) angiography, and intravascular ultrasound (IVUS). Though there is no consensus on which imaging modality is superior for TAVR planning, there is consensus that at least two modalities should be used for evaluation. At the Emory University Hospital in Atlanta, Ga., a bilateral lower extremity angiogram and noncontrast CT of the chest, abdomen, and pelvis are performed on all patients. The angiogram identifies stenoses, tortuosity, and aneurysms and allows for measurement of the true lumen diameter. Angiography should be done with digital subtraction angiography and a marker pigtail catheter (Figure 3–1). Rotational angiography has been advocated by some centers, but often a straight anteroposterior angiogram will allow for accurate calibration and measurements in both legs. The CT also helps confirm vessel size but primarily adds essential information about the location and extent of calcification in a vessel (Figures 3–2 and 3–3). For this reason it does not need to be done with contrast. Contrast CT can be performed in patients with adequate renal function and adds additional certainty of vessel size when using a work station that can rotate images in three dimensions. MR angiography has been used, but the resolution is not as good as CT and invasive angiography. IVUS has been used to confirm lumen size in patients, but measurements may be confounded by catheter bias in tortuous vessels. Figure 3–1 Proper technique for measuring the angiogram. Lower extremity angiography should be done using digital subtraction angiography. Zoom in on the area of interest and calibrate over two markers from the marker pigtail catheter (bracket) measuring leading edge to leading edge or lagging edge to lagging edge. Measure the diameter at the narrowest portion of the common iliac, external iliac (white arrow), and common femoral arteries on each side, making sure the measurement (white line) is exactly orthogonal to the direction of blood flow (red arrow). Figure 3–2 Excellent iliofemoral access for transcatheter aortic valve replacement in a patient with an aortic annulus measuring 21 mm (i.e., small transcatheter heart valve). Figure 3–3 No options for transcatheter aortic valve replacement (TAVR) from a transfemoral approach. The next step in patient selection is to determine whether the available sheath/delivery system will pass through the patient’s femoroiliac arteries. It has been determined that a noncalcified vessel will stretch up to 1 mm without rupture, although dissections can occur. SFARs above 1.05 have been associated with increased complications and mortality. A vessel with circumferential calcification will act like a rigid pipe and will not stretch beyond its nominal size without a significant risk of rupture. Noncircumferential calcification can cause significant drag on the delivery sheath, particularly in areas of high tortuosity such as the external iliac artery. Patients are often rejected for transfemoral approach if the external iliac arteries are calcified and the SFAR is greater than 1.0. Calcium in the common iliac arteries is very common in transfemoral patients; the area is often straight with an SFAR of less than 1.0 and thus of no consequence. Analysis of the angiography and noncontrast CT will usually reveal a preferred side for access, as well as the optimal part of the common femoral artery to puncture. The entry point into the common femoral artery should ideally be without calcification, even if this requires a “high” entry just distal to the inguinal ligament before the vessel dives into the pelvis. For patients over 100 kg, excessive scarring at or near the common femoral, or entry into the vessel at the area covered by the inguinal ligament, a surgical cut down should be considered. During preprocedure planning, the external or common iliac artery should be scrutinized for feasibility of an iliac conduit in case femoral access fails. If needed, this would be a Dacron graft anastomosis end-to-side to the external/common iliac artery, with the conduit externalized through the groin incision and the sheath inserted through the conduit. The minimum diameters accepted for 18F, 22F, and 24F sheaths are 6 mm, 7 mm, and 8 mm, respectively, in any part of the arterial tree.6 Alternatively, the patient should be considered for a transapical, transaortic, or subclavian approach. Accessing the common femoral artery through 1) an anterior wall puncture at 2) a noncalcified site with 3) adequate size to accommodate the sheath and 4) a location that allows for proximal compression/control of the vessel is paramount to successfully performing a completely percutaneous TAVR. Meeting these four criteria often leaves only a short segment of femoral artery that would be acceptable for access. Adjunctive imaging with femoral ultrasound or angiographic road mapping of the femoral iliac vessels is helpful. At Emory University Hospital, experience with angiographic road mapping has been developed (Figure 3–4). First the femoral artery is accessed, a 6F sheath is inserted, and a JR4 catheter is advanced to the level of the distal aorta. The ostium of the contralateral common iliac artery is engaged. The image intensifier is positioned over the contralateral femoral head, and a digital subtraction fluoroscopic road map image is taken using contrast injection. This creates an overlay of the contrast-filled lumen of the iliofemoral artery that remains on the screen during subsequent fluoroscopy. Figure 3–4 The roadmap image of the common femoral artery (A) and the corresponding fluoroscopic image (B) show the correct site of entry into the vessel (asterisk) below the pelvic rim and inguinal ligament at a compressible site without vessel calcification (white arrow on computed tomography inset image). The correct trajectory of the needle from a shallow angle and parallel to the common femoral artery course (white line) ensures anterior wall puncture and minimal vessel trauma.
Vascular Access for Structural Heart Disease
3.1 Arterial Access
3.2 Patient Selection
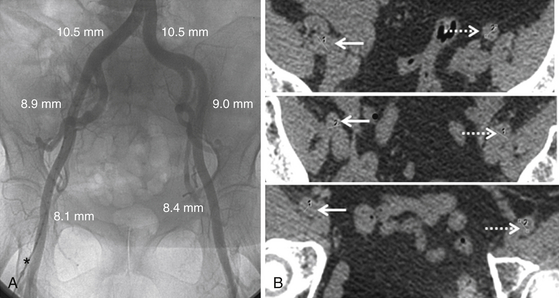
The lower extremity angiogram (A) shows the vessels’ lumen and tortuosity and allows precise measurement using a marker pigtail catheter (asterisk) for accurate calibration. Sequential axial cuts from the noncontrast computed tomography images (B) from the iliac to common femoral vessels on the right (solid arrows) and left (dashed arrows) show no calcification, indicating the vessel diameter will stretch up to 1 mm. The sheath–to–femoral artery ratio on the left is 0.83 for an 18F delivery system and approximately 1.0 for a 22F delivery system.
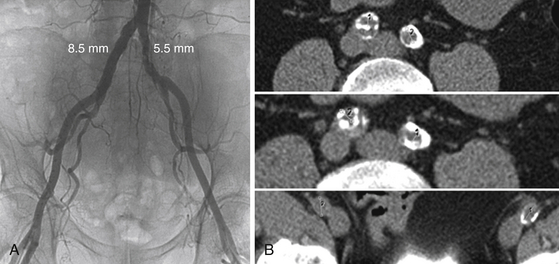
The lower extremity angiogram (A) shows obvious narrowing in the left common iliac artery, but the right common iliac artery appears to have an adequate diameter to accommodate an 18F to 22F sheath. Axial cuts from the noncontrast computed tomography (B) show a circumferentially calcified chronic dissection on the right that was not apparent on angiogram. The true lumen diameter is about 5 mm, illustrating the importance of using two different imaging modalities in concert when doing preprocedural planning. This patient had TAVR via a transapical approach.
3.3 Description of Technique
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Thoracic Key
Fastest Thoracic Insight Engine
