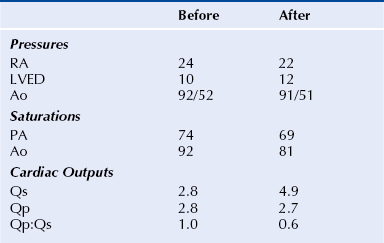
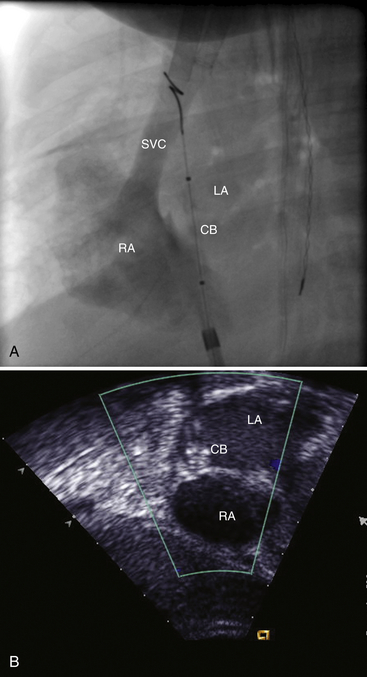
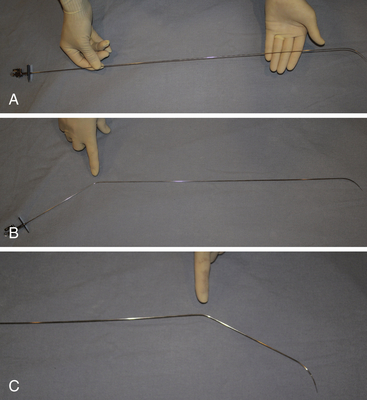
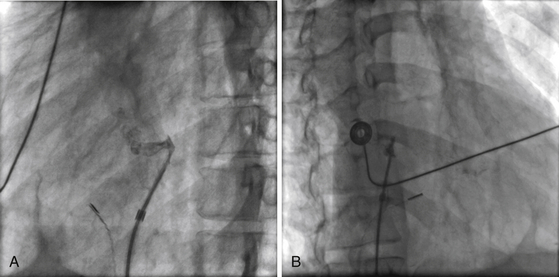
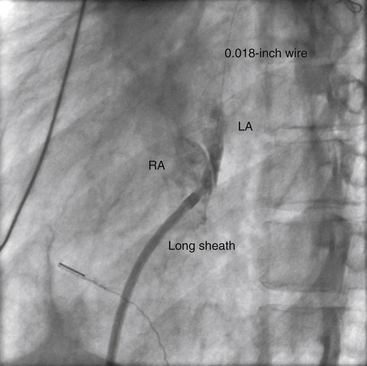
Chapter 21 Pulmonary hypertension (HTN) with end-stage pulmonary vascular disease results in right heart failure, low output, and death, with few therapeutic options other than lung transplantation. Opening of the atrial septum (most common), ventricular septum, or ductus arteriosus allows for right-to-left shunt decompression of the right heart volume and pressure at the expense of systemic desaturation. Opening of the atrial septum is the preferred approach in most patients because of the relative ease and safety. Despite the decrease in systemic saturation with opening of the atrial septum, left heart loading is augmented, enhancing cardiac output and improving peripheral O2 delivery.1–4 An optimal balance must be struck, because too much right-to-left shunting (too large a defect) results in profound desaturation with a marked decrease in tissue O2 delivery and exacerbates symptoms severely, to the point of death. After creation of a right-to-left shunt resulting in this physiologic tradeoff, clinical evidence has shown an improvement in symptoms,1,3,5 exercise tolerance,6–7 right ventricular function,5 and possibly improved survival.7–8 Clinical improvement can be sustained for many years.5 Interventional techniques now allow for an easy, safe nonsurgical option for this palliative therapy in patients. Nonsurgical opening of the atrial septum was first described in 1966 by Rashkind and Miller9 for palliative treatment of neonates with transposition of the great arteries, creating a left-to-right shunt at the atrial level to improve systemic saturations. This treatment remains in use for stabilization of neonates in anticipation of corrective surgical repair. The technique was expanded to treat older patients with transposition by the development of a “blade” catheter by Park et al in 1975.10 These techniques rapidly expanded to treat a variety of infants and children who had congenital defects with atrial septal restriction, such as mitral atresia and anomalous pulmonary venous return.11,12 In 1983, Rich and Lam13 applied the Park blade followed by a Rashkind atrial septostomy to a 28-year-old patient with end-stage pulmonary HTN for symptomatic palliation. Since then, newer techniques, including oversized static balloon dilation,14 cutting balloon dilation,15 stent implantation,16–18 and fenestrated device implantation19,20 have been used. This chapter details preferred techniques for atrial septal defect (ASD) creation, including cutting balloon dilation with large static balloon postdilation, stent implantation, and fenestrated device implantation. Patients receiving medical therapy for symptomatic end-stage pulmonary HTN who are awaiting transplant remain the primary group who benefits from opening of the atrial septum (Table 21–1). Syncope in the setting of severe pulmonary HTN carries a significant mortality risk. Wait times for lung transplantation remain long with limited survival to transplant. Patients who have pulmonary HTN but are not transplant candidates yet are symptomatic while receiving maximal medical therapy may also benefit from atrial septal opening with improvement in symptoms. Creation of a right-to-left shunt at the atrial level is also used in congenital cardiac patients with failing single ventricle physiology. These patients are typically surgically corrected with a total cavopulmonary connection, commonly referred to as a Fontan repair. In this setting the inferior vena cava (IVC) and superior vena cava (SVC) are directly connected to the pulmonary arteries for passive flow through the lungs. Cardiac or pulmonary failure results in IVC pressure elevation, causing a condition known as protein-losing enteropathy. When medical management fails, cardiac transplant is the only option. The creation of a communication between the Fontan circuit (physiologically the right atrium) and the left atrium with the development of a right-to-left shunt is an effective palliative treatment in some patients to improve protein-losing enteropathy. Clinical indications in this group of patients include the diagnosis of protein-losing enteropathy (low serum albumin and elevated stool α-1-antitrypsin) despite maximal medical therapy (e.g., diuretics, afterload reduction, pulmonary vasodilators) in patients whose saturations are greater than 90%. Lastly, patients with severe left ventricular (LV) dysfunction on ventricular assist device with left atrial (LA) HTN will benefit substantially from the creation of an atrial communication.21 In this setting, unlike others, the intent is for an adequate but transient atrial communication to allow a left-to-right shunt for LA decompression. The goal of defect patency is short, only a few weeks at most, while the ventricular-assist support is needed. To that end the favored technique for defect creation in this subgroup of patients would be cutting balloon with static balloon dilation to allow adequate immediate shunting, with a greater chance of spontaneous closure once the ASD is no longer needed. This technique has evolved as a result of the disappointingly high incidence of spontaneous closure of atrial septal communication with static balloon dilation alone.5,7,22 The technique is simple but requires an intact septum that is crossed solely with a transseptal needle or radiofrequency (RF) perforation wire, so the initial cutting balloon dilation will result in true scoring of the septum before large static balloon dilation (because of current limitation in maximum cutting balloon sizes at 8 mm diameter). The procedure is performed with the patient under moderate sedation with intracardiac echocardiography (ICE) guidance or if preferred, anesthesia with transesophageal echocardiography. Although echo guidance is not required, it does allow for more exact positioning of the defect creation and can be very useful in the setting of an existing patent foramen ovale (PFO) to assure the septum is crossed away from the PFO and not through it. Preferably the septum should be crossed inferior and posterior in the region of the fossa. Cutting balloon with large static balloon dilation through a PFO is ineffective at producing a persistent opening because of the distensibility of the PFO limiting the size of the tear in the septal tissue. This results in the creation of a small defect at most that has a high rate of spontaneous closure. A 7F or 8F transseptal sheath is placed in a femoral vein, preferably the right. The left vein can be used but may then necessitate adjusting the shape of the transseptal needle by adding a proximal bend of 30 to 45 degrees 10 to 15 cm along the needle from the hub (Figure 21–1). Monitoring of arterial pressure and saturations is needed to assess adequacy of the ASD size, so an arterial cannula is placed. For more accurate assessment, a 5F to 6F sheath is placed so direct LV pressure can be monitored. Complete right heart hemodynamics are obtained with a wedge or thermodilution catheter. Mixed venous saturations, both SVC and branch pulmonary artery (PA) samples, as well as arterial saturation (PaO2 as well if the patient is breathing supplemental O2) should be obtained at baseline and after ASD creation to determine shunting and guide defect size. Left heart pressure measurements with monitoring of left ventricle end-diastolic (LVED) pressure during ASD creation can also be helpful to assess adequacy of defect size. The hemodynamic changes shown in Table 21–2 with the creation of a 7-mm ASD (6-mm cutting balloon followed by a 7-mm diameter stent) in this 4-year-old child with severe pulmonary HTN but preserved LV function are typical. The acute creation of a significant right-to-left shunt increasing cardiac output results in a large decrease in aortic saturation with only a modest increase in LV filling pressure. This may not be the case in a diseased noncompliant left ventricle where creation of the same-sized ASD may result in a larger increase in LV filling pressure. Figure 21–1 Shaping transseptal needle to accommodate left femoral vein access. A, Normal needle, B, Needle shaped for LFV approach, and C, Additional bend if the right atrium is dilated. After initial hemodynamic assessment, transseptal puncture is performed centrally in the septum, preferably through the area of the fossa ovalis, if intact (Figure 21–2). If there is a PFO, the transseptal puncture should be performed slightly posterior and inferior, taking care not to slip thru the foramen but instead puncturing the septum primum. Once through the septum the dilator and sheath are advanced across the puncture into the left atrium, and pressure and saturation obtained. A shaped directional catheter such as a 5F JR4, multipurpose, or JB1 is used to position a stiff 0.014-inch wire or a stiff 0.018-inch wire in a left pulmonary vein, preferably the upper vein. An 8 mm × 2 cm cutting balloon is then advanced over the wire to the tip of the sheath in the left atrium. The sheath is pulled back to the right atrium and then advanced back to the septum, and a hand injection angiogram through the side-arm of the sheath is performed (Figure 21–3). This, together with ICE imaging, confirms septal location for exact balloon positioning. The balloon should be centered on the septum and fully inflated to a maximum of 8 atm using an insufflator to minimize risk of balloon rupture. Removal of a ruptured cutting balloon poses challenges with risks of vascular injury and blade embolization. As the balloon expands, a mild waist is often, but not always, seen (Figure 21–4 and Video 21–1, Video 21–1A, Video 21–1B). Figure 21–2 Anteroposterior (A) and lateral (B) angiographic views of the intact fossa ovalis as seen by contrast injection through transseptal sheath in the right atrium. Figure 21–3 Angiogram through side-arm of sheath, defining RA septal location before insertion of the cutting balloon. LA, Left atrium; RA, right atrium.
Atrial Septal Defect Creation
21.1 Patient Selection/Indications
21.2 Technique
Cutting Balloon/High-Pressure Balloon Dilation
![]() Repeat dilation should be performed with adjustment of balloon position if there is any concern that the balloon was not centered on the atrial septum based on angiographic and ICE landmarks.
Repeat dilation should be performed with adjustment of balloon position if there is any concern that the balloon was not centered on the atrial septum based on angiographic and ICE landmarks.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Atrial Septal Defect Creation