Fig. 7.1
Varicella virus. Round to hexagonal or “stop sign”-shaped, targetoid viral particles, cell culture media. Original magnification × 57000 (Courtesy of Dr. Richard Kradin and Dr. William Taylor, with permission from Saunders Elsevier)
7.5 Immunology
Initial exposure to VZV induces both humoral and cell-mediated immune responses. Primary VZV infection causes the production of IgM, IgG, and IgA antibodies that bind to many classes of viral proteins including glycoproteins, viral enzymes, and structural proteins. These antibodies either lyse infected cells by antibody-mediated cytotoxicity or neutralize the virus either directly or indirectly with the assistance of complement proteins. Antibodies against VZV do not provide protection against HZ and postherpetic neuralgia (PHN) although they may help to protect against primary infection or reinfection later in life at mucosal sites of inoculation (Miller et al. 1989; Zaia et al. 1983). It seems that increased levels of antibodies against VZV after the onset of HZ are associated with more severe disease and an increased risk of PHN (Weinberg et al. 2009).
A number of studies indicate that T cell-mediated immunity plays a crucial role against and recovery from varicella and HZ (Camitta et al. 1994; Gershon and Steinberg 1979; Haberthur et al. 2011; Vossen et al. 2004). Individuals with T cell-immune deficiency disorders have more severe disease than normal host, whereas individuals with agammaglobulinemia who are unable to produce VZV-specific antibodies acquire immunity against second episodes of varicella probably as a result of VZV-specific T cell-mediated immune response (Gershon and Steinberg 1979).
7.6 Clinical Features
Initial infection with VZV results in chicken pox (varicella), which is characterized by fever, posterior cervical lymphadenopathy, and rash on the skin and sometimes mucosa. The rash is centripetal (being concentrated on the scalp, face, and trunk rather than extremities) and pruritic. In addition, headache, malaise, and anorexia may be seen. The rash starts as macules and rapidly progresses to papules and then turns to characteristic vesicles and crusts, which then slough off in a week or two. VZV is an extremely infectious virus. Transmission among individuals follows either inhalation of respiratory aerosols from infected individuals or direct contact with skin lesions of patients with varicella or shingles. Incubation period is approximately 2 weeks. However, it could be longer (4 weeks) in subjects exposed to varicella and treated with specific VZV immunoglobulins. Contagiousness starts 2 days before the beginning of rash and persists until the last crop of vesicles has crusted. In countries where temperate climates exist, chicken pox is typically seen in children between 1 and 10 years of age and is a mild disease in immunocompetent children. However, the clinical course of infection acquired in adults (usually from countries where tropical climate exists) and immunocompromised children and adults is prolonged, more severe, and more commonly associated with such complications as pneumonia, encephalitis, cerebellitis, hepatitis, thrombocytopenia, and cutaneous bacterial superinfections (Beby-Defaux et al. 2009; Springfeld et al. 2009). Pneumonia occurs approximately in 1 out of 400 cases of chicken pox infection (Mandell et al. 2000). Pregnant women, developing fetuses, and neonates are at increased risk of mortality and morbidity from chicken pox.
Following the initial infection, VZV has the ability to become latent in neurons and satellite cells in the ganglia along the entire human neuroaxis including cranial nerve, dorsal root, and autonomic ganglia. As chicken pox vesicles can develop on any dermatome, all sensory ganglia with afferent fibers terminating in the vesicles may become latently infected. In addition to axonal retrograde transport, given the high virus load during chicken pox, neurons might also be infected via blood circulation (de Jong et al. 2000). Reactivation of latent VZV from these ganglia results in herpes zoster (shingles), a painful, unilateral vesicular eruption in a restricted dermatomal distribution and occurring predominantly in elderly or immunocompromised patients (Gnann and Whitley 2002). Reactivation is attributed to the natural immunosenescence (especially T cell-mediated immunity) that occurs with aging and other risk factors resulting in immunocompromise. These risk factors include underlying malignancy, disorders of cell-mediated immunity, use of chemotherapeutic and immunosuppressive medications for malignant neoplasms, and organ transplantations and HIV infection (Gnann and Whitley 2002; Johnson et al. 2007; McDonald et al. 2009). Once reactivated, the virus starts replicating within the sensory ganglion. It then spreads along the axons to the sensory nerve endings of the skin where it causes pruritic, painful, vesicular rash on an erythematous base in a localized dermatomal distribution. The rash does not usually cross the midline of the body and is usually limited to one to three dermatomes. The zoster pain may be mild to severe and has been variously described as stinging, tingling, aching, numbing, or throbbing and can be interspersed with quick stabs of severe agonizing pain (Katz et al. 2004). In addition, fever, headache, and malaise may be present. In immunocompetent patients, the lesions usually crust in a week or two and are no longer considered infectious. Zoster affects any level of neuroaxis, and patients may have additional symptoms depending on the dermatome involved. For example, uveitis, conjunctivitis, keratitis, and optic nerve palsies may be seen in herpes zoster ophthalmicus. Similarly, hearing loss, tinnitus, nystagmus, vertigo, and facial nerve palsies may be seen in herpes zoster oticus (Ramsay Hunt syndrome). The most commonly affected site is thoracic and lumbar dermatomes.
The complications of shingles include postherpetic neuralgia (PHN), meningitis, meningoencephalitis, cerebellitis, myelopathy, retinal necrosis, and zoster sine herpetica (pain without rash). The most common and debilitating complication of shingles is PHN, that is, constant, stabbing, or burning pain that persists for at least 3 months or longer (sometimes years) after resolution of rash. The risk of PHN grows in parallel with increasing age. Approximately 40 % of patients with shingles over age 50 experience PHN (Rogers and Tindall 1971; Vander Straten et al. 2001). Immunocompromised patients with shingles have more severe local dermatomal disease (cutaneous dissemination) and are at greater risk for viremia and visceral dissemination (Balfour 1988; Jantsch et al. 2011; Tomonari et al. 2003). Visceral dissemination could manifest itself as pneumonia, hepatitis, encephalitis, and disseminated intravascular coagulopathy.
7.7 Pathologic Changes
The histopathologic changes of VZV (chicken pox and shingles) lesions are similar to those of herpes simplex. In skin, the changes begin focally along the basal layer followed by the involvement of the entire epidermis by eventual formation of intraepidermal vesicles as a result of degeneration of keratinocytes and exudation of proteinaceous fluid. Some adjacent keratinocytes fuse, forming large multinucleated epithelial cells. Intranuclear, eosinophilic, ground-glass inclusions with irregular chromatin margination are noted in multinucleated and mononuclear keratinocytes. There are associated perivascular mild to moderate inflammatory cells predominantly composed of lymphocytes in the dermis.
VZV infection with visceral dissemination could induce similar findings in internal organs. Only pathologic changes in lung will be discussed here. On gross examination, the lungs are firm, heavy, and plum colored and reveal numerous necrotic and hemorrhagic lesions up to 1 cm in size throughout the lung parenchyma and visceral and parietal pleura (Feldman 1994; Raider 1971). Vesicles might be identified in the bronchi and trachea (Zaki and Paddock 2008a, b). Microscopically, the lungs show diffuse foci of necrotizing pneumonia involving alveolar walls (interstitial pneumonitis), bronchioles, and blood vessels. There are intra-alveolar collections of eosinophilic proteinaceous material, patchy hyaline membranes, inflammatory cells, nuclear debris, and hemorrhage (Fig. 7.2). VZV-infected cells with ground-glass intranuclear inclusions and irregular chromatin margination may be identified in the pneumocytes, capillary aendothelial cells, interstitial fibroblasts, or respiratory epithelial cells (Zaki and Paddock 2008a, b). Giant cell pneumonia may also be seen (Saito et al. 1989).
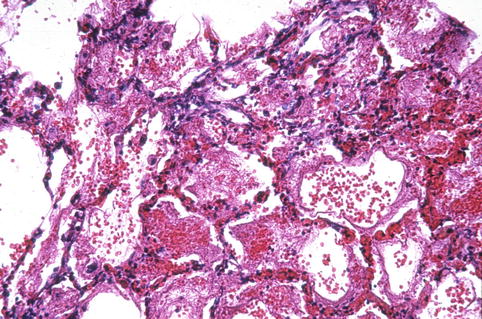

Fig. 7.2
Varicella-zoster pneumonia. Note extensive intra-alveolar hemorrhage (Courtesy of Dr. Richard Kradin, with permission from Saunders Elsevier)
In those patients who recover from VZV pneumonia, healing results in numerous, scattered, circumscribed fibrous nodules with calcification, which becomes visible on the chest imaging after an interval of 2–7 years (Floudas et al. 2008; Raider 1971). Therefore, numerous calcified opacities scattered throughout the lungs are virtually diagnostic of previous VZV pneumonia after the exclusion of histoplasmosis in geographic locations where histoplasmosis is endemic (Zaki and Paddock 2008a, b).
7.8 Diagnosis
VZV infection is usually diagnosed clinically based on the characteristic pruritic vesicular rash, which is widespread in chicken pox and localized in one to three dermatomal patterns with associated burning, stabbing pain in shingles. However, the location and appearance of the cutaneous lesions may be atypical (especially in immunocompromised subjects) and thus require laboratory confirmation. In addition, laboratory confirmation may be required in disseminated VZV infection without cutaneous lesions (usually immunocompromised host) and zoster sine herpetica (pain without rash). Serologic and virologic methods exist.
Serologic tests provide retrospective diagnosis as it requires testing of acute- and convalescent-phase serum specimens for IgG and/or IgM antibodies to VZV. These tests have limited value in acute setting as rapid confirmation of VZV infection is usually required for optimal treatment. The most important value of detecting IgG antibodies to VZV is to determine the immune status of individuals whose history of varicella infection or vaccination is unknown or equivocal. Serologic testing is used to determine the susceptibility to varicella and the need for immunization for health-care workers and immunocompromised patients at risk for reactivation. A plethora of serologic tests with different sensitivity and specificity such as FAMA (fluorescent-antibody membrane antigen assay), glycoprotein ELISA, and latex agglutination is available (Breuer et al. 2008; Sauerbrei and Wutzler 2006).
Virologic tests detect the presence of infectious virus, viral DNA, or viral protein in clinical specimens from a variety of sites. Direct fluorescent-antibody test on scrapings from active vesicular skin lesions allows rapid diagnosis of VZV lesions (Chan et al. 2001). Immunohistochemical stains using either polyclonal antibody to VZV IE63 protein or monoclonal antibody to VZV glycoprotein E also provide rapid and sufficiently accurate results on both smears (86.7 %) and formalin-fixed paraffin-embedded tissue specimens (92.3 %) (Nikkels et al. 1995). A variety of polymerase chain reaction tests (PCR) is available for identification of VZV DNA in clinical specimens such as cerebrospinal fluid, serum, blood, and bronchoalveolar lavage (Hobson-Peters et al. 2007; Markoulatos et al. 2000; Schmutzhard et al. 2004). PCR provides rapid and the most sensitive and specific confirmation of VZV. However, caution should be exercised because a positive result does not always prove that infectious virus is present. Similar to other herpes viruses, detection of VZV by PCR may be an accurate but incidental finding in many clinical situations. Therefore, correlation of PCR result with viral culture result, whenever is possible, is important. Viral culture is sometimes available for diagnostic use and still considered as reference standard for the identification of infectious VZV (Zaki and Paddock 2008a, b). However, VZV is fastidious and relatively difficult to recover from swabs of cutaneous lesions and many other sites depending on the clinical circumstances in cell culture (Gnann and Whitley 2002). Because the cytopathic effects on cell culture created by VZV are similar to those of HSV, isolate must be confirmed by staining with virus-specific antisera (Brinker and Doern 1993; Rogers and Tindall 1971).
7.9 Differential Diagnosis
Histopathologic differential diagnosis of VZV pneumonia includes pneumonia caused by HSV, adenovirus, parainfluenza virus, CMV, and measles. HSV pneumonia is histologically indistinguishable from VZV pneumonia by light and electron microscopy. Similar pattern of lung injury (multicentric, necrotizing, and hemorrhagic) and intranuclear inclusions in infected cells are seen in both VZV and HSV pneumonia. Distinction from HSV can be accomplished by immunohistochemical stains using antisera specific for VZV and HSV. VZV does not produce intracytoplasmic inclusions, which should be seen in parainfluenza virus, CMV, and measles. In addition, immunohistochemical stains with antisera specific for these viruses may prove to be useful for confirmation. Intranuclear inclusions of adenovirus are basophilic as opposed to eosinophilic, ground-glass inclusions seen in VZV infection. In addition, multinucleated epithelial giant cells are not seen in adenovirus infection. Again, in difficult cases, immunohistochemical stains using antisera specific for these viruses may be used to confirm the diagnosis (Zaki and Paddock 2008a).
7.10 Prevention
Varicella vaccines (Varivax and Zostavax) and varicella-zoster immunoglobulin are available for the prevention of chicken pox and shingles. Whether vaccine or varicella-zoster immunoglobulin is offered to a susceptible person with a history of varicella exposure will depend on the host. Varicella vaccine, which contains live attenuated virus, should not be given to immunocompromised patients. Advisory Committee on Immunization Practices (ACIP) recommends varicella-zoster immunoglobulin on the following patient groups (Centers for Disease Control and Prevention (CDC) 2012; Marin et al. 2007):
Immunocompromised patients (primary and acquired immunodeficiencies, immunosuppressive therapies, neoplastic diseases)
Neonates whose mothers have signs and symptoms of varicella around the time of delivery (i.e., 5 days before to 2 days after)
Premature infants born at ≥28 weeks of gestation who are exposed during the neonatal period and whose mothers do not have evidence of immunity
Premature infants born at <28 weeks of gestation or who weigh ≤1,000 g at birth and were exposed during the neonatal period, regardless of their mothers’ evidence of immunity status
Pregnant women
If varicella immunoglobulin is unavailable for the passive prevention of varicella for the above patient groups, acyclovir initiated within 3–7 days after exposure and continued through the incubation period might be effective in lessening disease severity and may prevent infection (Erard et al. 2007; Suga et al. 1993; Tarlow and Walters 1998).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


