Histopathology
(1) Cytomegaly
(2) Eosinophilic intranuclear Cowdry type B inclusions resembling an “owl’s eye”
(3) Amphophilic intracytoplasmic inclusions
Differential diagnosis
Herpes simplex
Varicella zoster
Adenovirus
Measles
Reactive pneumocytes
Radiographic findings
Bilateral nodules or reticular opacities
Pleural effusions (20 % of cases)
Treatment
Antiviral medications (ganciclovir, valganciclovir, acyclovir, valacyclovir, cidofovir, and foscarnet)
CMV-immune globulin (CMV-IVIG)
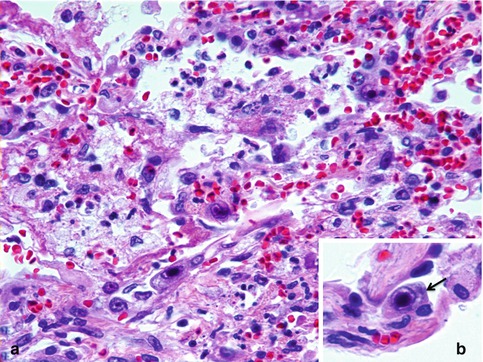
Fig. 5.1
CMV pneumonia. (a) Scattered alveolar cells with cytomegalic changes (H&E stain). (b) Note the characteristic eosinophilic intranuclear Cowdry type B inclusions resembling an “owl’s eye” with amphophilic intracytoplasmic inclusions (arrow) (inset, H&E stain)
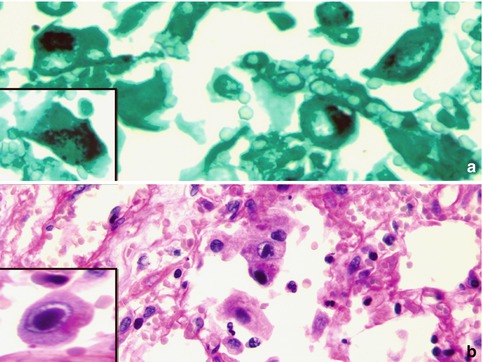
Fig. 5.2
CMV pneumonia. Multiple characteristic cytomegalic cells containing intracytoplasmic inclusions highlighted by (a) GMS and (b) PAS stains
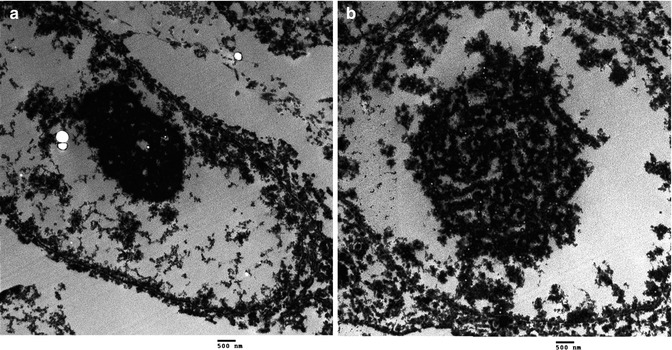
Fig. 5.3
CMV pneumonia. (a) and (b) Ultrastructural features of CMV in a postmortem lung with formation of electron-dense networks relative to the intranuclear inclusions composed of viral DNA and viral antigens (10000×)
CMV infection may have a variety of presentations such as rare scattered alveolar cells with cytomegalic changes, miliary disease with small nodules containing central hemorrhage and necrosis, interstitial pneumonitis, intra-alveolar hemorrhage, and diffuse alveolar damage with hyaline membranes (Beschorner et al. 1980; Dworniczak et al. 2003; Herry et al. 1996; Magro et al. 2005; Zhaori et al. 1996). Tracheobronchial ulcers do occur but to a lesser extent than in other herpetic infections, such as those due to herpes simplex. Co-infection with other pathogens can occur in CMV pneumonias, particularly with P. jiroveci. Therefore, thorough examination to rule out concurrent infection with bacteria, fungi, protozoa, as well as other viruses is important, especially in the presence of necrosis (Andrews 2004; Castagnola et al. 2004; Ljungman et al. 2002; Stagno et al. 1981).
5.8 Diagnosis
Definitive diagnosis requires laboratory confirmation (Table 5.2). The first laboratory method used to diagnose CMV was by urine cytology specimens (Montplaisir1972). The diagnostic gold standard for CMV is culture via shell vial method with human fibroblasts (Ison and Fishman 2005; Spector 1990). In addition to viral isolation, the diagnosis can be established by direct detection of intracellular viral antigens using immunohistochemistry (Fig. 5.4), or in situ hybridization of viral DNA by PCR (Ljungman et al. 2002; Strickler et al. 1990; Zhaori et al. 1996). Identification of serum CMV pp65 antigens detected in leukocytes may also be used as an antigenemia assay (Castagnola et al. 2004; de Maar et al. 2003; Ison and Fishman 2005; Ljungman et al. 2002). The viral load is determined by the antigenemia assay or quantitative PCR which correlates with symptomatic infection (Baldanti et al. 1998; Boeckh and Boivin 1998; Cunha 2010; de Maar et al. 2003; Egan et al. 1995; Guiver et al. 2001; Ison and Fishman 2005; Ljungman et al. 2002; Michaelides et al. 2001; van den Berg et al. 1989). Serologic studies identifying CMV-specific immunoglobulins have limited value as they are not useful in assessing active CMV infection, particularly after transplantation or in the immunosuppressed. However, CMV-specific immunoglobulins are useful in determining the risk of infection at the time of transplantation (de Maar et al. 2003). In immunocompetent individuals, antibodies develop to CMV over the first 4 to 7 weeks after infection. Either a single elevated IgM or a four-fold rise in IgG titers is required to diagnose a recent CMV infection (Cunha 2010; Ison and Fishman 2005).
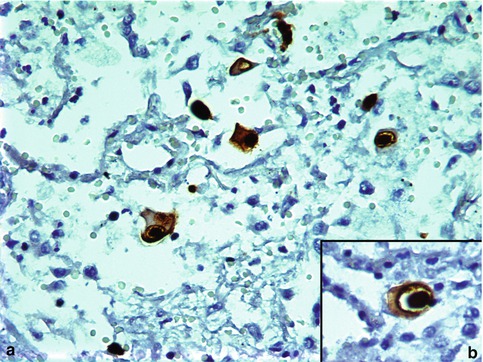
Table 5.2
Laboratory diagnosis of CMV
Direct detection of organisms | Histopathology |
Immunohistochemistry | |
Electron microscopy | |
CMV pp65 antigens | |
Serology | CMV-specific immunoglobulins |
Culture | Shell vial with human fibroblasts (gold standard) |
Molecular detection | In situ hybridization |
PCR |

Fig. 5.4
CMV pneumonia. CMV immunostain highlighting characteristic cytopathic cells (brown nuclear and cytoplasmic stain)
5.9 Differential Diagnosis
The inclusions may be confused with those of other herpes viruses such as herpes simplex virus (HSV) and varicella zoster virus (VZV), adenoviruses, or measles virus. Intranuclear inclusions are seen in VZV-, HSV-, and adenovirus-infected cells. Measles virus-infected cells contain basophilic intracytoplasmic inclusions. However, the combination of cytomegaly with both intranuclear and cytoplasmic inclusions are diagnostic features of CMV. Furthermore, these viruses can be distinguished by immunohistochemical stains using antisera specific for VZV, HSV, measles, and adenovirus. Reactive pneumocytes may contain enlarged nuclei but can be differentiated from CMV by a negative CMV immunostain.
5.10 Prevention
For CMV-naïve recipients, using seronegative donors or seronegative or leukocyte-reduced blood products aids in preventing CMV infection (Castagnola et al. 2004). In addition, prophylactic therapy with antiviral agents or through passive immunity with CMV-immune globulin may be useful, especially in the immunosuppressed (de Maar et al. 2003; Ison and Fishman 2005). Vaccines for CMV are not currently available: see below.
5.11 Treatment and Outcome
Treatment with CMV-reactive drugs such as ganciclovir, valganciclovir, acyclovir, valacyclovir, cidofovir or foscarnet, as well as CMV-specific immune globulin has improved mortality associated with CMV infection, particularly in immunocompromised individuals (Cunha 2010; de Maar et al. 2003; Ison and Fishman 2005; Uhlin et al. 2012). Quantitative assays should be used to monitor the response to therapy due to antiviral resistance. If an appropriate response to therapy does not occur within 2 weeks, molecular testing for antiviral resistance should be obtained (Ison and Fishman 2005). Specific DNA mutations have been identified leading to CMV resistance. The two most common mutations are found in the UL97 and UL54 regions. If resistance to ganciclovir has been identified, foscarnet or cidofovir is often effective (Baldanti et al. 2004; Kotton et al. 2010; Rodriguez et al. 2007; Uhlin et al. 2012). If CMV pneumonitis develops in the immunocompromised, despite prophylactic therapy, it is associated with a high mortality rate, particularly in bone marrow transplant patients (Castagnola et al. 2004; de la Hoz et al. 2002; de Maar et al. 2003; Ljungman 1995).
5.12 Vaccines
Currently, there are no licensed CMV vaccines available, but enormous progress has been made during the last decade with a variety of phase 1 and phase 2 clinical trials. Preliminary data shows that there is a reduction of infection/transmission using these vaccines (Sung and Schleiss 2010).
5.13 Clinicopathologic Capsule
A universal human pathogen, CMV is commonly associated with immunosuppression. CMV infection in immunocompetent individuals does occur but may be clinically silent. The lungs infected with CMV may show nodular foci of necrosis and/or hemorrhage. The microscopic damage inflicted on the lung is characteristic and distinctive. Main features are cytomegaly and the well-known eosinophilic intranuclear inclusion bodies that confer infected cells the so-called owl-eye appearance. The diagnostic gold standard for CMV is cultures via shell vial method with human fibroblasts, but often the diagnosis is suspected on basis of histopathology alone. Prevention can be achieved through passive immunity with CMV immunoglobulin. Therapy with drugs acting against CMV such as ganciclovir is effective and has improved mortality rates, but mutant strains have been recently indentified, leading to drug resistance. Licensed vaccines are not currently available.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


