Development of hospital-acquired anemia (HAA) during acute myocardial infarction may be related to processes of care and is associated with poor outcomes. Little is known about variation in the incidence of HAA across hospitals or the hospital characteristics associated with HAA. We studied 17,676 patients with acute myocardial infarction without anemia at admission, defining HAA as a hemoglobin decline below anemia diagnostic thresholds and moderate-to-severe HAA as a hemoglobin decline to <11 g/dl. We calculated median rate ratios (MRRs), the median value of the relative risk (RR) for HAA for 2 patients with identical characteristics presenting to 2 randomly selected hospitals, to identify variation in HAA adjusting for patient characteristics. Separate models were fit to test the association between hospital characteristics and HAA. HAA (57.5%) and moderate-to-severe HAA (20.1%) were common. The incidence of HAA varied substantially across hospitals and remained significant after multivariable adjustment (any HAA: MRR 1.09, 95% confidence interval (CI) 1.07 to 1.13; moderate-to-severe HAA: MRR 1.27, 95% CI 1.19 to 1.39). Adjusting for patient characteristics, teaching status (RR 0.91, 95% CI 0.84 to 0.97 vs nonteaching status), and region (Northeast vs Midwest: RR 1.10, 95% CI 1.01 to 1.19; West vs Midwest: RR 1.19, 95% CI 1.06 to 1.33, respectively) was associated with risk of HAA. Teaching status (RR 0.7, 95% CI 0.6 to 0.9 vs nonteaching status) and region (South vs Midwest: RR 1.3, 95% CI 1.0 to 1.5) were independently associated with moderate-to-severe HAA. In conclusion, we observed significant variability in the incidence of HAA across hospitals and found a lower risk of HAA at teaching centers, suggesting that qualitative studies of the relation between HAA and processes of care are needed to identify targets for quality improvement.
Hospital-acquired anemia (HAA), a new-onset anemia in patients hospitalized with normal hemoglobin, is common during acute myocardial infarction (AMI), frequently persists after discharge, and is associated with poor outcomes. The incidence of HAA also varies significantly across hospitals. Because HAA is associated with modifiable processes of care such as bleeding and diagnostic blood loss, this variability may reflect that different processes of care influence HAA rates. However, variability in the incidence of HAA across a broader sample of hospitals reflecting real-world practice, interhospital variability in the incidence of moderate-to-severe HAA (hemoglobin decline from normal to ≤11 g/dl), and the association between hospital characteristics and HAA have not been reported. Understanding the variability in HAA incidence across hospitals is the critical first step to identify practice patterns associated with lower HAA risk, which could ultimately lead to strategies to prevent and manage HAA. We studied a contemporary cohort of consecutive patients with AMI admitted to 57 hospitals to identify the incidence of HAA and moderate-to-severe HAA at each participating hospital, the variability in HAA after adjusting for patient characteristics, and the association of hospital characteristics with HAA.
Methods
We used the Cerner Health Facts database to study variability in the incidence of HAA and identify hospital characteristics associated with HAA. Health Facts captured deidentified data from the Cerner electronic medical record for consecutive patients admitted to participating hospitals between January 1, 2000 and December 31, 2008. Health Facts included hospital characteristics, patients’ demographics, medical history, and co-morbidities (using International Classification of Diseases, Ninth Revision, Clinical Modification codes), laboratory studies, in-hospital treatments, and complications.
A total of 78 hospitals contributed data to Health Facts, including a median of 219 patients (interquartile range 48, 1,030) over a median enrolment of 2.9 years (interquartile range 1.2, 5.3). Health Facts hospitals were mostly urban (88.5%), less frequently teaching hospitals (35.9%), represented all geographic regions of the United States (Northeast 38.5%, Midwest 25.6%, South 26.9%, and West 9%), and included a broad range of sizes (bed size: 1 to 99, 26.9%; 100 to 199, 20.5%; 200 to 299, 23.1%; 300 to 499, 17.9%; and ≥500 beds, 11.5%). Hospital teaching status was self-reported by sites participating in Health Facts. All data were deidentified before they were provided to the investigators, and an exemption from institutional review board review was obtained.
Consistent with prior work, we defined HAA using age-, gender-, and race-specific criteria described by Beutler and Waalen as a hemoglobin <13.7 g/dl for Caucasian men aged 20 to 59 years, <13.2 g/dl for Caucasian men ≥60 years, <12.9 g/dl for black men aged 20 to 59 years, <12.7 g/dl for black men ≥60 years, <12.2 g/dl for Caucasian women and <11.5 g/dl for black women. This classification is based upon analyses of large, contemporary cohorts and identifies anemia more accurately than the World Health Organization definition. In the absence of race-specific criteria defining HAA for patients of other racial backgrounds (<5% of Health Facts patients), we applied diagnostic criteria for whites. Patients were classified as having HAA if their initial hemoglobin was greater than the appropriate diagnostic threshold, but their lowest (nadir) hemoglobin during hospitalization fell below the threshold for anemia. Because moderate-to-severe HAA has been shown to be strongly associated with poor clinical outcomes, we also defined moderate-to-severe HAA (a hemoglobin decline from normal to ≤11 g/dl) as an outcome of interest for the present study. Chronic anemia was defined as an admission hemoglobin value below the aforementioned thresholds for the diagnosis of anemia.
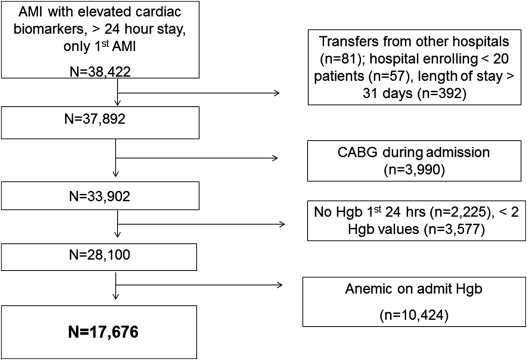
We included all patients hospitalized with a primary discharge diagnosis of AMI, determined by International Classification of Diseases, Ninth Revision, Clinical Modification diagnostic codes 410.xx, confirmed by at least 1 elevated cardiac biomarker (troponin or creatine kinase-MB), and who remained hospitalized for at least 24 hours. We limited our cohort to each patient’s first AMI hospitalization ( Figure 1 ). We excluded patients known to have been transferred from other hospitals or from hospice care. To maximize generalizability, we excluded patients from hospitals treating <20 patients with AMI during the study period and patients with lengths of stay >31 days. We also excluded patients who underwent coronary bypass during the index hospitalization, because both the etiology and outcomes of HAA are different in these patients. Finally, patients who did not have hemoglobin checked within the first 24 hours of the hospitalization had fewer than 2 hemoglobin assessments, and patients who had anemia on admission were excluded, yielding a final analytic cohort of 17,676 patients with AMI and without anemia on admission from 57 hospitals. To contrast the variability in HAA, we also analyzed the variability in the rate of chronic anemia among the 10,423 patients in the Health Facts database who were anemic at the time of admission but otherwise met inclusion criteria for this study.
The proportion of patients with any HAA and the proportion of patients with moderate-to-severe HAA were calculated for each hospital. Baseline patient characteristics, laboratory values, in-hospital treatments, and in-hospital complications were compared between patients with no HAA, mild HAA, and moderate-to-severe HAA. We presented categorical data as frequencies and compared differences between groups using chi-square tests. Continuous variables are reported as the mean ± SD or median (interquartile range), and differences between groups were compared using one-way analysis of variance.
We used generalized linear models that modeled hospital as a random variable to generate shrinkage estimates of the incidence of HAA and moderate-to-severe HAA for each Health Facts hospital to account for less precise estimates of small hospitals’ HAA rates by adjusting hospitals’ rates of HAA toward the overall population mean. We also calculated median rate ratios (MRRs) to identify the variability in HAA incidence after adjusting for patient characteristics. This method identifies the median value of the relative risk (RR) of moderate-to-severe HAA for 2 patients with identical covariates presenting to all possible pairs of Health Facts hospitals. For example, an MRR of 1.30 would indicate that if 2 patients with identical covariates each presented to different hospitals, one would experience a 30% greater risk for HAA at the higher risk hospital. These models adjusted for age, gender, race, ST-segment elevation myocardial infarction versus non-ST-segment elevation myocardial infarction, initial creatinine, diabetes, hypertension, heart failure, prior myocardial infarction, in-hospital acute renal failure, cardiogenic shock, cardiac catheterization or percutaneous coronary intervention, heparin, glycoprotein IIb/IIIa inhibitor, bivalirudin, thienopyridine, aspirin, and thrombolytic use.
To identify hospital characteristics that were independently associated with either any HAA or moderate-to-severe HAA after adjusting for patient characteristics, we used hierarchical Poisson regression with robust error variance, because the outcome of interest is prevalent. Hospital characteristics collected in Health Facts included bed size, hospitals’ teaching status, urban versus rural practice setting, and region of the country (Midwest, South, East, and West). Potential confounders were identified a priori based upon clinical experience and the prior literature, including the same variables used in the models described to assess variability in HAA rates.
Missing data were minimal (<5%) and were assumed to be missing at random. To include these patients in multivariable modeling, we used sequential regression imputation to impute missing values with IVEware software (Survey Research Center, Institute for Social Research, University of Michigan, Ann Arbor, Michigan). Statistical analyses were conducted using SAS, version 9.2 (SAS Institute, Cary, North Carolina) and R (version 2.11.1, R Core Development Team, Vienna, Austria).
Results
HAA was common, developing in 10,166 patients (57.5%). Mild HAA developed in 6,615 (37.4%) and moderate-to-severe HAA developed in 3,551 (20.1%) patients. Patient characteristics by HAA status are listed in Table 1 .
| Variable | HAA | p Value | ||
|---|---|---|---|---|
| None (n = 7,510) | Mild (n = 6,615) | Moderate-to-Severe (n = 3,551) | ||
| Minimum hemoglobin (g/dl) | 13.88 ± 1.03 | 12.20 ± 0.69 | 9.78 ± 1.14 | <0.001 |
| Admission hemoglobin (g/dl) | 15.07 ± 1.30 | 14.30 ± 1.09 | 13.64 ± 1.15 | <0.001 |
| Final hemoglobin (g/dl) | 14.15 ± 1.12 | 12.55 ± 0.88 | 10.67 ± 1.04 | <0.001 |
| Decrease in hemoglobin from admission to minimum (g/dl) | −1.19 ± 0.95 | −2.10 ± 1.03 | −3.86 ± 1.64 | <0.001 |
| Transfusion | 3 (0.0) | 4 (0.1) | 171 (4.8) | <0.001 |
| Age (yrs) | 63.2 ± 14.5 | 66.0 ± 14.3 | 71.9 ± 13.0 | <0.001 |
| White | 6,442 (85.8) | 5,872 (88.8) | 2,933 (82.6) | <0.001 |
| Women | 2,650 (35.3) | 2,001 (30.2) | 2,481 (69.9) | <0.001 |
| Length of stay (days) | 3.1 (2.3, 4.4) | 3.9 (2.8, 5.7) | 6.5 (4.1, 10.1) | <0.001 |
| Acute renal failure | 204 (2.7) | 306 (4.6) | 513 (14.4) | <0.001 |
| Peripheral arterial disease | 138 (1.8) | 155 (2.3) | 101 (2.8) | 0.003 |
| Diabetes mellitus | 1,793 (23.9) | 1,773 (26.8) | 1,134 (31.9) | <0.001 |
| Prior coronary bypass | 372 (5.0) | 270 (4.1) | 97 (2.7) | <0.001 |
| Cardiogenic shock | 104 (1.4) | 201 (3.0) | 336 (9.5) | <0.001 |
| Chronic kidney disease | 276 (3.7) | 372 (5.6) | 454 (12.8) | <0.001 |
| Chronic lung disease | 839 (11.2) | 796 (12.0) | 558 (15.7) | <0.001 |
| End-stage renal disease | 52 (0.7) | 72 (1.1) | 142 (4.0) | <0.001 |
| Dyslipidemia ∗ | 3,984 (53.0) | 3,072 (46.4) | 1,054 (29.7) | <0.001 |
| Heart failure | 1,391 (18.5) | 1,604 (24.2) | 1,377 (38.8) | <0.001 |
| Hypertension | 4,240 (56.5) | 3,593 (54.3) | 1,793 (50.5) | <0.001 |
| Prior percutaneous coronary intervention | 521 (6.9) | 402 (6.1) | 109 (3.1) | <0.001 |
| Prior myocardial infarction | 533 (7.1) | 376 (5.7) | 131 (3.7) | <0.001 |
| Current smoking | 2,761 (36.8) | 1,874 (28.3) | 483 (13.6) | <0.001 |
| Prior stroke/transient ischemic attack | 162 (2.2) | 158 (2.4) | 167 (4.7) | <0.001 |
| ST-elevation myocardial infarction | 3,072 (40.9) | 3,055 (46.2) | 1,547 (43.6) | <0.001 |
| Angiotensin-converting enzyme inhibitor | 4,460 (59.4) | 4,096 (61.9) | 2,221 (62.6) | <0.001 |
| Thienopyridine | 5,260 (70.0) | 4,664 (70.5) | 2,379 (67.0) | <0.001 |
| Angiotensin receptor blocker | 453 (6.0) | 443 (6.7) | 301 (8.5) | <0.001 |
| Aspirin | 6,480 (86.3) | 5,824 (88.0) | 3,091 (87.1) | 0.008 |
| β blocker | 6,292 (83.8) | 5,665 (85.6) | 3,049 (85.9) | 0.002 |
| Diuretics | 2,126 (28.3) | 2,250 (34.0) | 1,935 (54.5) | <0.001 |
| Glycoprotein IIb/IIIa antagonist | 3,774 (50.3) | 3,439 (52.0) | 1,702 (47.9) | <0.001 |
| Heparin | 3,622 (48.2) | 3,602 (54.5) | 1,957 (55.1) | <0.001 |
| Statin | 5,232 (69.7) | 4,601 (69.6) | 2,301 (64.8) | <0.001 |
| Thrombolytics | 242 (3.2) | 333 (5.0) | 184 (5.2) | <0.001 |
| Warfarin | 611 (8.1) | 684 (10.3) | 507 (14.3) | <0.001 |
| Coronary angiography | 5,487 (73.1) | 4,697 (71.0) | 2,236 (63.0) | <0.001 |
| In-hospital percutaneous coronary intervention | 4,204 (56.0) | 3,766 (56.9) | 1,719 (48.4) | <0.001 |
| Bleeding event † | 171 (2.3) | 265 (4.0) | 499 (14.1) | <0.001 |
| Bleeding site (percent of bleeding events) | <0.001 | |||
| Miscellaneous site | 119 (69.6) | 182 (68.7) | 290 (58.1) | |
| Gastrointestinal bleeding | 38 (22.2) | 62 (23.4) | 196 (39.3) | |
| Intracranial bleeding | 14 (8.2) | 21 (7.9) | 13 (2.6) | |
| Bivalirudin | 359 (4.8) | 297 (4.5) | 129 (3.6) | 0.023 |
| Admission creatinine (g/dl) | 1.00 (0.90, 1.20) | 1.10 (0.90, 1.30) | 1.10 (0.90, 1.50) | <0.001 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


