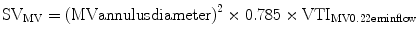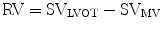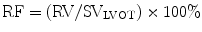, James L. JanuzziJr.2, James L. JanuzziJr.3, Aidan W. Flynn4, Praveen Mehrotra4, Igor F. Palacios5 and Igor F. Palacios6, 7
(1)
Harvard Medical School Interventional Cardiology, Cardiology Division, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA
(2)
Harvard Medical School, Boston, USA
(3)
Cardiac Intensive Care Unit, Cardiology Division, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA
(4)
Harvard Medical School Cardiology Division, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA
(5)
Harvard Medical School, Boston, USA
(6)
Knight Catheterization Laboratory, Boston, USA
(7)
Interventional Cardiology, Cardiology Division, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA
Abstract
Valvular heart disease is a growing public health concern – as our population ages, the prevalence of valvular heart disease will only rise. The expansive topic encompasses numerous disease entities, complex hemodynamics, invasive and noninvasive testing, and involved management decisions. In addition, perhaps more so than any other topic in cardiology, the diagnosis and management of valvular heart disease is dependent on the history and physical examination. Such complexity makes valvular heart disease a prime topic for cardiology board examinations.
Abbreviations
AR
Aortic regurgitation
AS
Aortic stenosis
AV
Aortic valve
BAV
Percutaneous balloon aortic valvuloplasty
CSA
Cross sectional area
DFP
Diastolic filling period
DT
Deceleration time
HR
Heart rate
LV
Left ventricular
LVOT
Left ventricular outflow tract
MR
Mitral regurgitation
MS
Mitral stenosis
MVA
Mitral valve area
MVG
Mitral valve gradient
MVR
Mitral valve replacement
NYHA
New York Heart Association
PHT
Pressure half-time
PMV
Percutaneous mitral valvuloplasty
PR
Pulmonic regurgitation
PS
Pulmonic stenosis
RA
Right atrial
RV
Right ventricular
TR
Tricuspid regurgitation
TS
Tricuspid stenosis
VTI
Velocity time integral
Introduction
Valvular heart disease is a growing public health concern – as our population ages, the prevalence of valvular heart disease will only rise. The expansive topic encompasses numerous disease entities, complex hemodynamics, invasive and noninvasive testing, and involved management decisions. In addition, perhaps more so than any other topic in cardiology, the diagnosis and management of valvular heart disease is dependent on the history and physical examination. Such complexity makes valvular heart disease a prime topic for cardiology board examinations.
Aortic Stenosis
Etiology
The most common cause of aortic stenosis is calcific degeneration [1].
Calcific aortic stenosis (AS) was historically felt to be due to age-related degeneration; however, it is due to an active process similar to atherosclerosis that includes lipid deposition, inflammation, and active calcification [2–6]. Severe calcific AS most frequently presents in the sixth to seventh decades of life.
Bicuspid aortic valves are prevalent in 1–2 % of the general population, predominantly men. Severe AS due to a bicuspid aortic valve can occur early in life, but most frequently presents in the fifth and sixth decades of life [7, 8].
Rheumatic AS rarely occurs in the Western world and is most often associated with concomitant mitral pathology.
Pathophysiology and Hemodynamics
Aortic stenosis (AS) causes an obstruction to left ventricular outflow tract, resulting in a fixed cardiac output and concentric left ventricular (LV) hypertrophy in compensation for left ventricular pressure overload.
Left ventricular hypertrophy occurs in order to maintain normal wall stress (σ) which is proportional to the LV pressure (P) and radius (r) and inversely related to wall thickness (T) as dictated by LaPlace’s law:
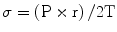
Worsening AS leads to progressive LV hypertrophy that in turn leads to diastolic dysfunction and myocardial oxygen supply–demand mismatch.
Low-flow aortic stenosis
In patients with AS and LV systolic dysfunction, AV leaflet opening may be reduced due to a low stroke volume, not severe AS (pseudostenosis).
Identification of pseudostenosis is frequently performed by assessing the severity of AS during low-dose dobutamine infusion. An increase in the AVA during dobutamine infusion signifies pseudostenosis; whereas, an increase in AV gradients with a constant AVA suggests true AS [9].
Assessment
History (See Chap. 1 for important details)
AS is typically asymptomatic until valvular stenosis is severe.
Symptom development in a patient with moderate AS may suggest the presence of underlying coronary artery disease
The hallmark of AS is the classical triad of dyspnea on exertion, chest heaviness, and dizziness with exertion.
These symptoms do not develop simultaneously, and in many cases, only one of the three is present.
Physical examination (see Chap. 1 for important details)
The physical examination of the patient with AS is very important to remember.
Most important hallmarks of severe aortic stenosis on physical examination include:
Reduction in the amplitude and velocity of the carotid upstrokes
Diminution or entire loss of the second heart sound
Mid-to-late peaking nature to the systolic murmur
The murmur of AS radiates to the carotids, sometimes associated with a thrill.
Radiation may also occur across the precordium
The murmur increases with squatting, decreases with standing and handgrip
This helps to differentiate it from hypertrophic cardiomyopathy.
Other findings include an opening click in patients with a bicuspid valve, as well as the murmur of aortic regurgitation.
Echocardiography
Transthoracic Doppler echocardiography is the standard method for quantifying the degree of aortic stenosis (Table 16-1).
Table 16-1
Severity of aortic valve stenosis
Aortic sclerosis
Mild
Moderate
Severe
Aortic jet velocity (m/s)
≤2.5 m/s
2.6–2.9
3.0–4.0
>4.0
Mean gradient (mmHg)
–
<20
20–40
>40
AVA (cm2)
–
>1.5
1.0–1.5
<1.0
Indexed AVA (cm2/m2)
>0.85
0.60–0.85
<0.6
Velocity ratio
>0.50
0.25–0.50
<0.25
The velocity (V) across the stenotic aortic valve on echocardiography can be used to estimate the peak pressure gradient across the valve (ΔP) by use of the Bernoulli equation:
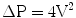
The principle of conservation of mass dictates that flow within the left ventricular outflow tract (LVOT) must be the same as flow through the aortic valve. Hence, the aortic valve area (AVA) can be calculated using the continuity equation:
where VTI is the velocity time integral and CSA is cross sectional area.
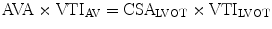
Cardiac catheterization
Invasive assessment of AS severity is recommended when noninvasive tests are inconclusive or discordant with clinical findings [10].
During cardiac catheterization, the mean gradient across the aortic valve (MVG) is measured and used to calculate the AVA using the Gorlin formula [11]:
where CO (ml/min) is cardiac output, HR (bpm) is heart rate, SEP (s) is systolic ejection period, and MVG is mean valve gradient.
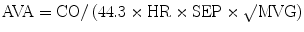
The Hakki equation simplified the Gorlin formula for routine use in clinical practice [12]:
where CO (L/min) is cardiac output and MVG is mean valve gradient.
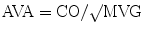
Natural History
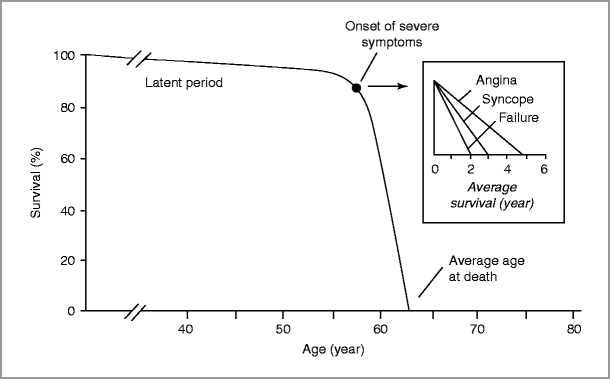
Aortic stenosis typically progresses slowly over decades (latent phase) with an average rate of progression of 0.1–0.2 cm2/year [13, 14], although more rapid progression is seen with heavily calcified valves [15].
Aortic sclerosis progresses to severe AS in some, but not all, individuals. Progression from sclerosis to stenosis over a 5-year interval was observed in approximately 9 % of the Cardiovascular Health Study (CHS) population, all of whom were older than 65 years [16].
Once symptoms due to severe AS develop, survival worsens.
Sudden death occurs in the setting of severe AS, whether or not symptoms have developed; however, sudden death is rare in asymptomatic AS patients (<1 %/year) [10].
Other complications of AS include left ventricular dysfunction, worsening mitral regurgitation from annular dilation, heart failure, and conduction disease from erosion of calcium at the level of the aortic annulus into the upper septum and affecting either the atrioventricular node (first degree block) or the bundles.
Concomitant ascending aortic dilation is present in patients with bicuspid aortic valves (independent of the degree of stenosis or regurgitation).
Dilation of the ascending aorta in a patient with a bicuspid valve is not due to “post-stenotic” turbulence of blood flow; rather it is due to an inherited weakness of the medial smooth muscle integrity.
Aortic dissection is a dreaded complication in this scenario.
Management of AS
Pharmacologic
Patients with asymptomatic AS have outcomes similar to normal, adult controls.
Consequently, with the exception of serial screening for worsening valve stenosis, no further management for asymptomatic AS is needed.
There is little in the way of medical therapy for slowing progression of AS.
For patients with dilated ascending aorta, use of beta blockers and possibly vasodilators (such as angiotensin receptor blockers) is recommended to retard progression of dilation and reduce risk for dissection.
Aortic regurgitation should be treated as below
Aortic valve replacement
The current indications for aortic valve replacement (AVR) are listed in Table 16-2.
Table 16-2
Indications for aortic valve replacement for aortic stenosis
Class
Aortic stenosis
Symptomatic patients with severe AS
Class I
Patients with severe AS undergoing cardiac surgery
Class I
Asymptomatic patients with severe AS and LV systolic dysfunction (LV EF <50 %)
Class I
Patients with moderate AS undergoing cardiac surgery
Class IIa
Asymptomatic patients with severe AS and abnormal response to exercise
Class IIb
Asymptomatic patients with severe AS if there is a high likelihood of rapid progression or surgical delay at the time of symptom development
Class IIb
Patients with mild AS and evidence that progression may be rapid
Class IIb
Asymptomatic patients with extremely severe AS (AVA <0.6 cm2, mean gradient >60 mmHg, jet velocity >5.0 m/s) if expected operative mortality <1 %
Class IIb
Surgical AVR is the standard of care for patients with severe, symptomatic AS and in those with severe AS and LV systolic dysfunction.
Valve replacement in this setting alleviates symptoms and results in substantial reduction in mortality. Survival after surgical AVR approximates that of age-matched controls [10].
Surgery should not be considered for asymptomatic patients with severe AS unless there is a need for other cardiac or aortic surgery or if there is a high likelihood of rapid progression [10].
In patients with low ejection fraction due to the AS, AVR may lead to considerable improvement in ventricular function, in contrast to this situation in aortic regurgitation.
In patients with a dilated ascending aorta at the time of surgery, replacement of the aneurysm may be indicated, particularly if the size is >40 mm or if there is a family history of aortic dissection.
In patients at high-risk for surgical AVR, transcatheter aortic valve replacement (TAVR) has recently been introduced and approved by the United States Food and Drug Administration (FDA) based on finding from the PARTNER I trial [21].
Percutaneous aortic valvuloplasty
Percutaneous balloon aortic valvuplasty (BAV) is a procedure in which a balloon is inflated across the stenotic AV in order to increase valve opening [22].
Aortic valvuloplasty is effective for severe AS in children and adolescents; however, its efficacy is limited in adults with calcific AS due to short-lived and only modest clinical benefits [10].
In the current era, BAV is used as a bridge to surgical or transcatheter AVR in unstable patients with severe AS and as a palliative procedure in those in whom other definitive therapies are not feasible.
Aortic Regurgitation
Etiology
Aortic regurgitation (AR) develops due to abnormalities in either the aortic root or the valve leaflets.
Acute and chronic AR are distinct clinical entities which will be considered independently.
There are several causes of chronic AR, the most common of which is dilation of the aortic root and AV annulus. Several other etiologies are also noteworthy (Table 16-3).
Table 16-3
Causes of aortic regurgitation
Leaflet abnormalites
Chronic regurgitation
Bicuspid aortic valve
Calcific valve disease
Rheumatic valve disease
Myxomatous valve disease
Rheumatoid arthritis
Nonbacterial thrombotic endocarditis
Systemic lupus erythematosus
Pharmacologic agents
Acute regurgitation
Endocarditis
Iatrogenic leaflet damage
Ruptured leaflet fenestration
Blunt chest trauma
Abnormalities of the aorta
Chronic regurgitation
Marfan syndrome
Bicuspid aortic valve disease
Hypertensive aortic dilation
Familial aortic aneurysm
Cardiovascular syphilis
Ankylosing spondylitis
Other systemic inflammatory disorders
Acute regurgitation
Aortic dissection
Acute AR is less common and usually due to infective endocarditis, aortic dissection, or trauma [10].
Pathophysiology and Hemodynamics
In AR, the fundamental insult is LV volume overload. The extent of overload and regurgitation depends on the regurgitant orifice, the diastolic pressure gradient across the AV, and the duration of diastole [23].
Chronic aortic regurgitation
In response to chronic volume overload, eccentric LV hypertrophy develops in order to increase LV end-diastolic volume. Thus, the compliant LV can accommodate the increased volume load without an increase in end-diastolic pressures [10].
Eccentric hypertrophy also results in a larger stroke volume in the setting of preserved LV function; thereby, maintaining effective forward stroke volume.
With progressive chronic AR, LV systolic dysfunction ultimately ensues. This further increases LV end-diastolic volume and pressure, resulting in marked LV dilatation and dysfunction.
Acute aortic regurgitation
With acute AR, forward cardiac output drops substantially as the LV does not have the opportunity to adapt to the volume load of AR [24].
In severe cases, hemodynamic instability and cardiogenic shock ensue. Mean left atrial pressure rises, resulting in pulmonary edema as well.
The pre-existence of pressure-overload in which the LV is small and non-compliant results in more dramatic decompensation as these patients possess a steeper diastolic pressure-volume relationship [10, 24].
Myocardial perfusion pressure may also diminish as LV end-diastolic pressure approaches the diastolic aortic pressure, resulting in subendocardial ischemia.
Assessment
History
Until left ventricular end diastolic pressure begins to rise, patients with AR are frequently asymptomatic.
Symptoms may relate to exaggerated cardiac output with increased stroke volume, including a sense of pounding in the chest.
Dyspnea is an ominous sign, implying a rise in left ventricular end diastolic pressure with the onset of heart failure.
Physical examination (see Chap. 1 for important details)
The exam of the patient with AR is important to understand.
Vital sign hallmarks of significant AR include a pulse indicative of both elevated cardiac output and diastolic “run off”.
The ‘Waterhammer’ pulse is an exaggerated upstroke most notable in the carotid pulse.
The pulse pressure in chronic AR is usually wide.
The pulse pressure may re-narrow in the context of a high left ventricular end diastolic pressure, such as in heart failure.
The point of maximal impulse is usually displaced in severe chronic AR but may be normally located in acute AR.
The diastolic murmur of AR.
Most often musical (“blowing”), but may be harsh when due to aortic leaflet eversion, tearing, or perforation.
The murmur of AR typically radiates along the left sternal border and is best heard with the patient sitting up and leaning forward with a full expiration.
If the murmur radiates along the right sternal border, it is suggestive of an ectatic aortic root, such as which occurs in syphilis.
With worsening aortic regurgitation and onset of ventricular failure, the murmur may shorten.
Very significant AR is frequently accompanied by a mitral diastolic rumble (the “Austin-Flint” murmur) due to impingement on the mitral valve by the regurgitant volume.
Other stigmata of AR are discussed in Chap. 1 but the classical findings of chronic AR (such as head bobbing, to-and-fro murmurs over the vascular structures, or higher leg blood pressures compared to arm) are less evident or absent in acute AR.
Echocardiography
Echocardiography can be invaluable in identifying the etiology and severity of AR and allows assessment of the ascending aorta.
Doppler and color flow echocardiography is used to grade the severity of AR using several parameters (Table 16-4) [10, 25].
Table 16-4
Measures of aortic regurgitation severity
Parameter
Mild
Moderate
Severe
Jet width/LVOT (%)
<25
25–65
>65
Vena contracta (cm)
<0.3
0.3–0.6
>0.6
Pressure half-time (ms)
>500
200–500
<200
Regurgitant volume (mL/beat)
<30
30–60
>60
Regurgitant fraction (%)
<30
30–50
>50
Regurgitant orifice area (cm2)
<0.10
0.1–0.3
>0.30
The ratio of AR jet width/area to LVOT diameter/area correlate with angiographic AR severity.
The time required for the AV pressure gradient in diastole to fall by half (“pressure half-time”) also correlates with AR severity; however, the ability of this measure to distinguish between grades of AR is limited. Shorter pressure half-times are associated with more severe AR.
Regurgitant orifice area (ROA) is a robust measure of AR severity that is calculated by dividing the regurgitant volume by regurgitant flow (VTIAR) [23]:
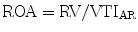
Other findings consistent with severe AR include premature closure of the mitral valve, mitral valve fluttering, reversed doming of the anterior mitral valve leaflet, and holodiastolic flow reversal in the descending aorta.
Cardiac catheterization
Invasive assessment of AS severity is recommended when noninvasive tests are inconclusive or discordant with clinical findings [10].
Invasive hemodynamic measurements can be helpful in evaluating patients with mixed aortic valve stenosis and regurgitation.
Supravalvular aortography can be used to grade AR severity based on the degree of contrast regurgitation into the LV (Table 16-5) [27].
Table 16-5
Seller’s criteria for grading aortic regurgitation by angiography
Grade of regurgitation
1
Small amount contrast enters LV in diastole and is cleared with each beat
2
More contrast fills LV with faint opacification of the entire LV
3
LV is well opacified with contrast density equal to the ascending aorta
4
Complete and dense opacification of the LV on the first beat with contrast density greater than the ascending aorta
Natural History
Acute AR is often a medical emergency that requires immediate intervention.
Asymptomatic patients with preserved LV function and without severe LV dilatation possess a good prognosis.
The rate of progression to LV dysfunction and/or symptom development is only 4.3 %/year and the rate of sudden death only 0.2 %/year [10].
The guidelines for valve replacement surgery in the asymptomatic patient are listed in Table 16-6.
Table 16-6
Indications for aortic valve replacement for aortic regurgitation
Class
Symptomatic patients with severe AR
Class I
Asymptomatic patients with severe AR and LV systolic dysfunction (LV EF ≤50 %)
Class I
Asymptomatic patients with severe AR undergoing cardiac surgery
Class I
Asymptomatic patients with severe AR with severe LV dilatation (EDD >75 mm or ESD >55 mm)
Class IIa
Patients with moderate AR undergoing cardiac surgery
Class IIb
Asymptomatic patients with severe AR with LV dilatation (EDD >70 mm or ESD >50 mm) when there is evidence for progressive LV dilatation, declining exercise tolerance, or abnormal hemodynamic response to exercise
Class IIb
Management
Pharmacologic
Chronic aortic regurgitation
Vasodilators are the primary therapy for asymptomatic patients with chronic severe AR.
Vasodilators such as hydralazine, nifedipine and felodipine increase cardiac output and reduce the regurgitant fraction [28, 29].
Therapy with angiotensin-converting enzyme inhibitors (ACE-I) reduces end-diastolic volume if doses sufficient to reduce systemic blood pressure are administered [10, 30].
Long-term therapy with vasodilators is only indicated for those without an indication for valve replacement or in those who cannot undergo surgery [10].
Short-term therapy can be instituted for hemodynamic optimization prior to surgical AVR [10].
Acute aortic regurgitation
Medical therapy for acute severe AR should be used solely to maintain hemodynamic stability prior to surgical AVR.
Intravenous vasodilators, such as nitroprusside, should be used to reduce afterload and LV end-diastolic pressure and to augment cardiac output.
Inotropic agents can also be used to further increase cardiac output if needed, but are generally not useful.
Surgical management of AR (Table 16-6)
Surgical AVR is a class I indication for symptomatic patients with severe AR regardless of LV dimensions and function [10].
Surgery is a class I indication for asymptomatic patients with LV dysfunction (ejection fraction ≤50 %) and those asymptomatic patients with severe AR undergoing concomitant cardiac surgery.
AVR may be considered (Class II) in asymptomatic patients with severe LV dilatation.
Despite high operative risk, clinical outcomes with AVR in patients with NYHA class IV symptoms and/or severe LV dysfunction (LV ejection fraction ≤25 %) are better than with medical therapy alone [31].
Surgical aortic valve repair (rather than replacement) for AR is feasible, especially in those with bicuspid aortic valves or those with AR due to cusp prolapse.
Mitral Stenosis
Etiology
Most common cause of mitral stenosis (MS) is rheumatic heart disease due to previous rheumatic fever.
Rheumatic MS involves mitral valve leaflet thickening and calcification, commissural fusion, chordal fusion, and ultimate obstruction [32].< div class='tao-gold-member'>Only gold members can continue reading. Log In or Register to continue

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree