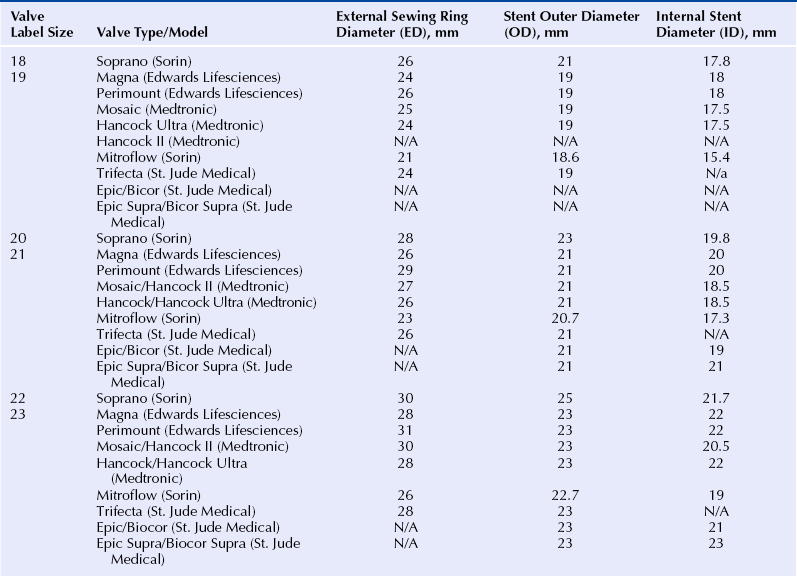
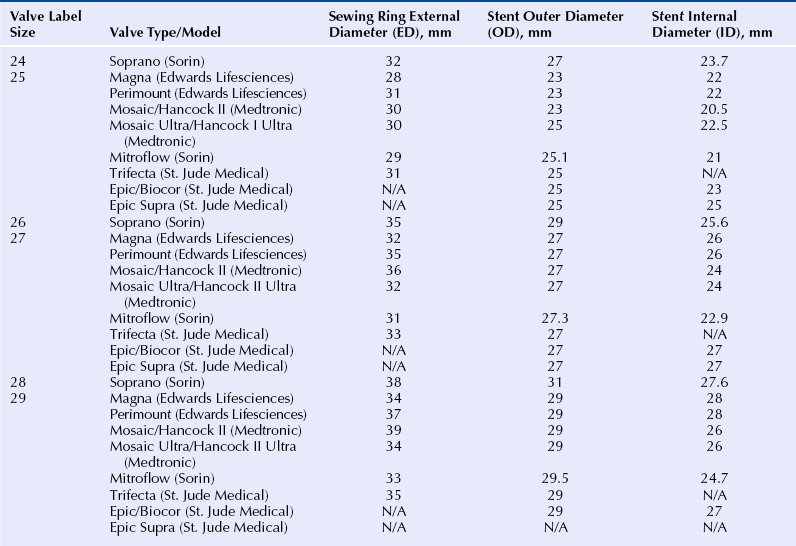
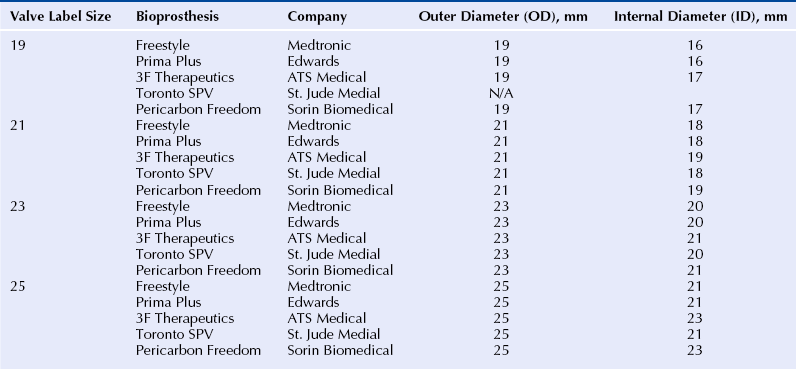
Chapter 13 • Transcatheter valve implantation is feasible in failing surgical aortic, mitral, pulmonic and tricuspid bioprostheses. • Access may be transarterial (femoral, subclavian, axillary), transvenous (femoral, subclavian, jugular), transapical, transatrial, or direct transaortic. • Paravalvular regurgitation (PR) must be distinguished from valvular regurgitation, because it will not respond to valve-in-valve (VIV) implants. • The specific model and the labeled (external) diameter of the bioprosthesis must be confirmed. • The internal diameter of the bioprosthesis must be determined from the manufacturer and noninvasive imaging. • The transcatheter valve size must match or exceed the internal diameter of the surgical bioprosthesis. • VIV implantation in very small surgical bioprostheses may lead to high transvalvular gradients. • The radiographic and echocardiographic appearance of the surgical bioprosthesis posts and sewing ring should be understood. • Transcatheter valves depend largely on anchoring within the sewing ring, not the stent posts. • The durability of VIV implants is unknown at the present time. Reoperative replacement of failing bioprosthetic heart valves carries a higher risk of morbidity and mortality than the initial valve replacement procedure. An alternative to reoperation is transcatheter VIV implantation. Transcatheter VIV implantation has been successfully performed for failing surgical bioprostheses in the aortic, mitral, pulmonic, and tricuspid positions. This chapter covers the practical challenges, selection criteria, techniques, and outcomes of VIV implantation. Bioprosthetic valves are derived from animal tissue (xenograft or heterograft) or from human tissue (homograft). The most commonly used biologic materials are porcine aortic valve and bovine pericardial tissue. In stented bioprostheses the leaflet tissue is suspended from a frame made of various alloys or plastics. The stent is seated on a basal ring, which may be circular or saddle shaped. Freedom from bioprosthetic valve failure is reportedly 70% to 90% at 10 years and 40% to 70% at 15 years.1–5 Failure may occur because of stenosis, regurgitation, both of these, or a paravalvular leak. A major contributor to bioprosthetic failure is calcification, which involves an interaction between phospholipids, glutaraldehyde collagen cross-linking, and circulating calcium.6 Younger age at implantation, diabetes, renal failure, and abnormalities of calcium metabolism such as hyperparathyroidism are risk factors for accelerated valve calcification. Structural failure may also occur as a result of a leaflet tear, which may occur in the absence of calcification.7 Typically this occurs in the area of the commissures, where the leaflet stress is highest. Whereas growth of host tissue onto the valve frame is part of a natural healing process, proliferative overgrowth with pannus restricts leaflet mobility. Additional mechanisms of structural valve failure include infective endocarditis and valve thrombosis, both contraindications for VIV procedures. Transcatheter VIV implantation may be considered in patients with failing surgical bioprostheses caused by severe regurgitation, stenosis, or both, regardless of surgical valve position. Significant PR has to be excluded, because it does not respond to VIV therapy. Endocarditis and valve thrombosis are generally considered contraindications. The lack of long-term durability data restricts VIV procedures to patients at high or prohibitive surgical risk. Furthermore, the internal diameter of the surgical bioprosthesis limits the size of the transcatheter heart valve (THV), which is of concern when dealing with a very small bioprosthesis. Patients need to undergo thorough screening to determine the most appropriate access site that allows safe and effective THV delivery. Comorbidities that may influence life expectancy should be taken into account before pursuing with VIV treatment. Surgical bioprostheses are usually referred to according to their labeled size, a number that roughly corresponds to the diameter in mm of the aortic annulus as determined by a surgeon intraoperatively. This labeled size does not refer to the internal diameter of the valve, which is the most important diameter to consider in VIV treatment (Figure 13–1). In most cases the labeled size corresponds to the outer diameter of the stent; however, in the case of stentless valves it typically corresponds to the external diameter of the valve. Unfortunately valve size labeling varies by manufacturer and is not standardized; moreover, different models of valves with the same reported label size (external diameter) may have different internal diameters. Figure 13–1 Schematic representation of a stented surgical bioprosthesis. A surgical operative report or patient valve implant card should be obtained to verify the specific manufacturer, model, and labeled size of the valve actually implanted. The internal diameter should then be ascertained from the manufacturer. The dimensions of a range of commonly implanted surgical bioprostheses are found in Tables 13–1 to 13–3. The nominal external diameter of the THV for VIV implantation should match or exceed the internal diameter of the surgical bioprosthesis. Undersizing the THV may lead to intervalvular regurgitation or embolization. Excessive oversizing of the THV may result in underexpansion of the THV frame within the surgical bioprosthesis and lead to compromised hemodynamics and leaflet durability. In the authors’ experience, underexpansion up to 20% of the nominal THV diameter is generally acceptable.8 However, sizing considerations should not only take into account the internal prosthesis diameter, but also the nature of surgical bioprosthetic valve failure. Highly calcified, stenotic valves or those with prominent pannus may have smaller internal diameters. Imaging findings from computed tomography or transesophageal echocardiography should be taken into account when assessing THV sizing. However, their accuracy and reproducibility have not been tested for this indication. TABLE 13–1 Valve Dimensions for Selected 18- to 23-mm Surgical Bioprostheses, as per Manufacturer Product Information (Reprinted with permission from Elsevier; Gurvitch, R, Cheung, A, Ye, J, et al: Transcatheter valve-in-valve implantation for failed surgical bioprosthetic valves. J Am Coll Cardiol 58:2196-2209, 2011.) TABLE 13–2 Valve Dimensions for Selected 24- to 29-mm Surgical Bioprostheses, as per Manufacturer Product Information (Reprinted with permission from Elsevier; Gurvitch, R, Cheung, A, Ye, J, et al: Transcatheter valve-in-valve implantation for failed surgical bioprosthetic valves. J Am Coll Cardiol 58:2196-2209, 2011.) TABLE 13–3 Valve Dimensions for Selected Stentless Valves, as per Manufacturer Product Information (Reprinted with permission from Elsevier; Gurvitch, R, Cheung, A, Ye, J, et al: Transcatheter valve-in-valve implantation for failed surgical bioprosthetic valves. J Am Coll Cardiol 58:2196-2209, 2011.) 1. The Edwards SAPIEN and SAPIEN XT: The Edwards SAPIEN THV (Figure 13–2) is a balloon-expandable valve composed of a stainless steel frame with bovine pericardial leaflets. The inflow of the frame is covered with a fabric cuff to provide an annular seal. It comes in two sizes with external diameters of 23 mm and 26 mm. The successor is the SAPIEN XT valve (Figure 13–2), which has a cobalt chromium frame, bovine pericardial leaflets and is manufactured in four sizes with external diameters of 20 mm, 23 mm, 26 mm, and 29 mm. These valves have been successfully used for VIV procedures in the aortic, mitral, pulmonic, and tricuspid positions. Unlike self-expanding valves, balloon-expandable valves are suitable for both retrograde (e.g., transapical for mitral bioprostheses) as well as the antegrade (e.g., transjugular for tricuspid bioprostheses) procedures. For transarterial or transvenous approaches the valve is compressed onto the low profile NovaFlex delivery catheter and introduced through an 18F to 20F sheath (or 16F expandable sheath). Figure 13–2 Edwards SAPIEN Transcatheter Heart Valve (left) and Edwards SAPIEN XT Transcatheter Heart Valve (right) balloon expandable valves. 2. The Medtronic CoreValve: The CoreValve system is composed of a self-expandable nitinol frame with porcine pericardial leaflets (Figure 13–3). The system is manufactured in four sizes with external annular diameters of 23 mm, 26 mm, 29 mm, and 31 mm. The self-expanding valve frame is deployed by retraction of a restraining sheath. The system may only be used in a retrograde transarterial orientation (transfemoral, subclavian, axillary, or transaortic), and the long frame prevents its use in mitral, tricuspid, and pulmonary prostheses. A potential advantage of this valve is that the leaflets are mounted relatively high (supraannular) within the stent frame, with the potential for larger valve orifice when implanted in small diameter surgical bioprostheses. (See also Chapter 7.) Figure 13–3 The CoreValve™ System. 3. The Melody valve: The Melody transcatheter valve (Figure 13–4) is composed of a bovine jugular venous valve sutured within a platinum iridium stent. The system is manufactured in four sizes, including 23 mm, 26 mm, 29 mm, and 31 mm. The valve is designed for use in pulmonary circulation, mostly to treat dysfunctional right ventricular outflow tract conduits or other pulmonary bioprostheses in patients with congenital heart disease. It has also been used for the tricuspid,9 aortic,10 and mitral10 positions. The functioning of this valve in a low-pressure environment is well established, but long-term durability in a high-pressure environment may be questionable. (See also Chapter 8.) Figure 13–4 The Melody™ Transcatheter Pulmonary Valve. 4. The Inovare valve: The Inovare valve (Braile Biomédica, Brazil) consists of a balloon expandable cobalt–chromium stent with bovine pericardial leaflets (Figure 13–5). It was approved for transapical native aortic valve implantation in November, 2011, in Brazil. It is only available with a transapical delivery system, although a transfemoral system is under development. There is only very limited experience with VIV procedures11 (personal communication Valter Lima MD, Porto Alegre, Brazil). Very similar in appearance to the SAPIEN valve, it may offer a lower cost alternative to the other valves. Figure 13–5 The Inovare Braile Biomédica Valve.
Valve-in-Valve Therapy
13.1 Key Points
13.2 Causes of Bioprosthetic Valve Failure
13.3 Patient Selection and Indications for Valve-in-Valve Therapy
Dimensions of Surgical Bioprosthetic Heart Valves
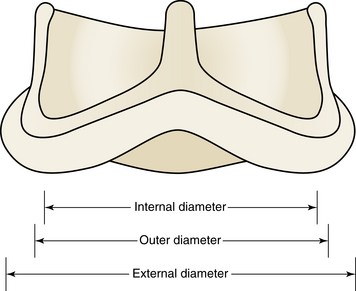
The internal, outer, and external diameters all represent different dimensions of surgical bioprostheses. Whereas surgeons have to conform the size of the bioprosthesis to the diameter of the native valve annulus, operators performing valve-in-valve procedures have to fit the transcatheter heart valve to the internal diameter of the bioprosthesis. (Reprinted with permission from Elsevier; Gurvitch, R, Cheung, A, Ye, J, et al: Transcatheter valve-in-valve implantation for failed surgical bioprosthetic valves. J Am Coll Cardiol 58:2196-2209, 2011.)
13.4 Transcatheter Heart Valves and Device Selection
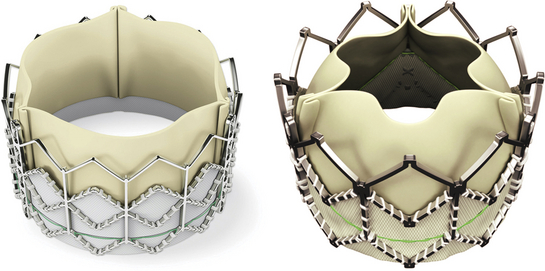
These balloon-expandable bovine pericardial tissue valves may be implanted antegrade or retrograde for valve-in-valve procedures in the aortic, mitral, pulmonic, and tricuspid positions. Accurate deployment mostly requires rapid ventricular pacing. (Images published with permission of Edwards Lifesciences LLC, Irvine, Calif. Edwards SAPIEN and Edwards SAPIEN XT are trademarks of Edwards Lifesciences Corporation.)
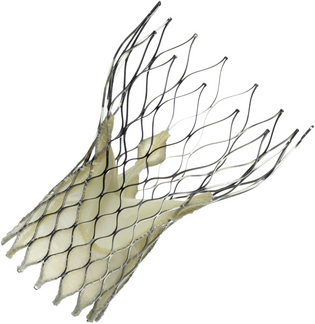
The CoreValve is a self-expandable nitinol porcine pericardial tissue valve. It was the first self-expandable valve used for a valve-in-valve procedure.18 It is suitable only for the aortic position and only via a retrograde approach (e.g., transfemoral, transsubclavian, or direct transaortic). (Courtesy of Medtronic, Minn.)
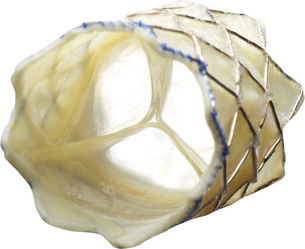
The Melody valve was the first transcatheter valve ever implanted in a human. It is most commonly used for stenotic or regurgitant right ventricle–pulmonary artery conduits in patients with congenital heart disease. The valve consists of a platinum iridium stent with a bovine jugular vein valve sewn inside. (Courtesy of Medtronic, Minn.)
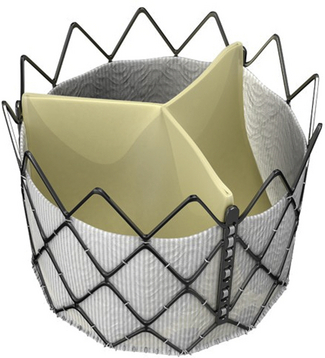
The Inovare Braile Biomédica Valve is a balloon-expandable cobalt–chromium pericardial leaflet valve that had been used in about a dozen valve-in-valve procedures at the time of publication. (Courtesy of Braile Biomédica, Brazil.)![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Valve-in-Valve Therapy
