Acute kidney injury (AKI) after transcatheter aortic valve implantation (TAVI) is frequent and associated with adverse outcomes. We aimed to identify the incidence and risk factors for AKI after TAVI using the updated Valve Academic Research Consortium–2 classification criteria. We performed a retrospective analysis of 300 consecutive patients undergoing TAVI using either Edwards SAPIEN XT or CoreValve bioprostheses at our medical center. Change in serum creatinine from base line to 48 to 72 hours after TAVI was used to define AKI stages 1 to 3. The final study cohort included 251 patients. Overall incidence of AKI was 16.7% (42 of 251); of which, stage 1 AKI was 15.1% (38 of 251), stage 2 AKI was 1.6% (4 of 251), and none had stage 3 AKI or required hemodialysis. All-cause mortality at 30 days and 1 year was greater among patients with AKI (9.5% vs 1%, p <0.01% and 25.7% vs 12.3%, p = 0.041, respectively). Despite greater volume of contrast media used in Edwards SAPIEN versus CoreValve (162 ml vs 142 ml, p = 0.02), there was no difference in the incidence of AKI between the 2 valve types (23.7% vs 15.5%, p = 0.238) or when comparing larger (29 to 31 mm) versus smaller size valves (23 to 26 mm; 17.7% vs 16.1%, p = 0.745). AKI was associated with chronic kidney disease, history of peripheral vascular disease, blood transfusion, and higher EuroSCORE (p <0.05 for all). In conclusion, according to the new Valve Academic Research Consortium–2 classification, 1 in every 6 patients in our cohort developed AKI after TAVI (most were stage 1 AKI). AKI was associated with increased mortality. No difference in AKI incidence was observed between different types and sizes of bioprostheses used.
Patients undergoing transcatheter aortic valve implantation (TAVI) are older and have a high prevalence of chronic kidney disease (CKD) and are therefore at increased risk of developing acute kidney injury (AKI). In recent reports, AKI has been observed in 12% to 41% of patients undergoing TAVI and was associated with a 4× greater postprocedural mortality. The Valve Academic Research Consortium–2 (VARC-2) has recently updated the classification criteria for AKI after TAVI ; this consortium has proposed a 3-stage modified classification based on the known RIFLE criteria (Risk, Injury, Failure, Loss and End-stage kidney disease). No study has yet reported the prevalence of AKI after TAVI using these updated criteria. The objective of our study is to report the rate of AKI after TAVI using the updated VARC-2 criteria at our institute and evaluate the effect of the different prosthesis types on AKI.
Methods
The data for the present study were collected in the period from March 2009 to September 2012, at the division of interventional cardiology at the Tel Aviv Medical Center, Tel Aviv, Israel. Informed consent was obtained from each patient as approved by the Institutional Ethics Committee. The diagnosis of aortic stenosis was based on clinical, echocardiographic, and hemodynamic criteria. Suitability and eligibility for TAVI was determined by our heart team. During the study period, 300 consecutive patients undergoing TAVI were enrolled.
Because the classification of AKI requires laboratory data at baseline and 48 to 72 hours after procedure, we have limited the present analysis to patients with adequate laboratory data and those without end-stage renal disease. Therefore, for the present analysis, we excluded 45 patients with incomplete laboratory data and 4 patients treated with chronic hemodialysis. Thus, the final study group is comprised of 251 patients.
Two types of aortic valve prostheses were routinely implanted at that period in our institution: Edwards SAPIEN XT prosthesis (Edwards Lifesciences, Irvine, California) and the CoreValve aortic valve prosthesis (Medtronic, Minneapolis, Minnesota). For all procedures, a senior interventional cardiologist was responsible for all aspects of the case, including the administration of contrast media. Patients requiring a coronary angioplasty before TAVI were treated 3 to 4 weeks before the TAVI procedure to minimize the risk of developing contrast-induced AKI. The contrast medium used in all TAVI procedures was iodixanol (Visipaque, GE healthcare, Ireland), which is an iso-osmolar contrast medium that was demonstrated to be associated with less nephrotoxicity compared with high osmolar contrast media commonly in use. All patients had an overnight hydration before the procedure (normal saline solution for 24 hours, at a rate of 100 ml/hour, beginning 12 hours before the scheduled procedure) and administration of oral N-acetylcysteine (1,200 mg × 2/day for 2 days starting 24 hours before the procedure).
Valve type and size were planned before the procedure according to preprocedural echocardiographic, tomograhic, and angiographic parameters and at the discretion of the senior interventional cardiologist. The available valve sizes during the study period for the Edwards SAPIEN XT prosthesis were 23 and 26 mm and for the CoreValve prosthesis 23, 26, 29, and 31 mm. For the present analysis, we defined larger prosthesis sizes as 29 and 31 mm and smaller sizes as 23 and 26 mm.
Serum creatinine level was measured at baseline (1 day before the procedure), on the procedure day (after an overnight hydration), and at 48 to 72 hour after the procedure. If there was >1 measurement available, the greater serum creatinine value was included in the analysis. AKI was defined as stage 1, 2, or 3 according to VARC-2 AKI classification ( Table 1 ). If patients required renal replacement therapy (RRT), they were classed as stage 3 as indicated by VARC-2. For the present analysis, CKD was defined as baseline estimated glomerular filtration rate of <60 ml/min/1.73 m 2 .
| Stage | Criteria |
|---|---|
| Stage 1 | Increase in serum creatinine to 150% to 199% of baseline Or Increase of ≥0.3 mg/dl (26.4 mmol/L) Or Urine output <0.5 ml/kg/h for >6 but <12 h |
| Stage 2 | Increase in serum creatinine to 200% to 299% of baseline Or Urine output <0.5 ml/kg/h for >12 but <24 h |
| Stage 3 | Increase in serum creatinine to ≥300% of baseline Or Increase of serum creatinine of ≥4.0 mg/dl (354 mmol/L) with an acute increase of at least 0.5 mg/dl (44 mmol/L) Or Urine output <0.3 ml/kg/h for >24 h Or Anuria for >12 h |
All data are displayed as mean ± SD for continuous variables and the number (percentage) of patients in each group for categorical variables. The Student t test and chi-square test were used to evaluate the statistical significance between continuous and categorical variables, respectively. Odds ratios (OR) were calculated with a confidence interval (CI) of 95%. Binary logistic regression models were performed with the dependent variable AKI adjusted to the following variables: previous myocardial infarction (MI), hypertension, congestive heart failure (CHF), diabetes mellitus (DM), peripheral vascular disease (PVD), EuroSCORE, contrast media, chronic renal failure, and blood transfusion. These variables were found to be associated with AKI after TAVI in previous reports and in our analysis. Cox proportional hazards models for all-cause mortality were adjusted to age, gender, DM, hypertension, PVD, CHF, chronic renal failure, previous MI, previous coronary artery bypass graft surgery, and EuroSCORE. Follow-up was defined as the time from the TAVI procedure to the date of mortality or February 8, 2013. All of the analyses were considered significant at a 2-tailed p value of <0.05. The SPSS statistical package 20 was used to perform all statistical evaluation (SPSS, Chicago, Illinois).
Results
The final study cohort included 251 patients (37.5% men) at a mean age of 83.2 ± 5.13 years. The baseline characteristics of the study population are summarized in Table 2 . A high prevalence of co-morbidities and previous vascular disease was observed on top of the underlying valvular disorder, with 85.7% patients suffering from hypertension, 31.5% DM, 35.9% CHF, and 58.6% coronary heart disease. Mean logistic calculated EuroSCORE was 26.1 ± 12.
| Variable | All (n = 251) | AKI | p | |
|---|---|---|---|---|
| Yes (n = 42) | No (n = 209) | |||
| Preprocedural clinical data | ||||
| Age (yrs) | 83.2 ± 5.1 | 83.5 ± 5.8 | 83.2 ± 5 | 0.73 |
| Men | 94 (37) | 14 (28) | 80 (38) | 0.36 |
| DM | 79 (31) | 14 (33) | 65 (31) | 0.77 |
| Dyslipidemia ∗ | 195 (77) | 34 (81) | 161 (77) | 0.57 |
| Hypertension | 215 (85) | 39 (93) | 176 (84) | 0.14 |
| Height | 161.7 ± 8.7 | 160.45 ± 8.2 | 162 ± 8.8 | 0.3 |
| Weight | 71 ± 14 | 69.8 ± 14 | 71.4 ± 14.4 | 0.54 |
| Body mass index (kg/m 2 ) | 27 ± 4.9 | 26.8 ± 5.2 | 27.1 ± 4.9 | 0.78 |
| Creatinine clearance † (ml/min/1.73 m 2 ) | 62.2 ± 18 | 63.8 ± 17 | 54.3 ± 19 | 0.002 |
| PVD | 25 (10) | 8 (19) | 17 (8) | 0.03 |
| Stroke | 20 (9) | 5 (12) | 15 (7) | 0.3 |
| CHF | 90 (35) | 17 (40) | 73 (35) | 0.4 |
| History of coronary artery disease | 147 (58) | 29 (69) | 118 (56) | 0.13 |
| Previous myocardial infarction | 44 (17) | 9 (21) | 35 (17) | 0.46 |
| Previous percutaneous coronary intervention | 115 (46) | 23 (55) | 92 (44) | 0.2 |
| Previous coronary artery bypass grafting | 44 (17) | 8 (19) | 36 (17) | 0.77 |
| Chronic obstructive pulmonary disease | 59 (23) | 9 (21) | 50 (24) | 0.72 |
| EuroSCORE | 26.1 ± 12 | 30.09 ± 13.1 | 25.3 ± 12.6 | 0.032 |
| Chronic atrial fibrillation | 44 (17) | 9 (21) | 35 (16) | 0.46 |
| Pulmonary hypertension | 152 (61) | 31 (74) | 121 (57) | 0.054 |
| Preprocedural echocardiographic data | ||||
| Aortic valve area (cm²) | 0.6 ± 0.19 | 0.69 ± 0.189 | 0.69 ± 0.19 | 0.9 |
| Peak pressure gradient (mm Hg) | 77 ± 23 | 77.7 ± 22.3 | 77.9 ± 23.4 | 0.95 |
| Mean pressure gradient (mm Hg) | 47.3 ± 15.1 | 47.1 ± 15 | 47.3 ± 15.2 | 0.99 |
| Ejection fraction (%) | 55.4 ± 7.8 | 53.5 ± 9.7 | 55.8 ± 7.4 | 0.07 |
| Procedure-related data | ||||
| Contrast volume (ml) | 145.6 ± 44.3 | 156.1 ± 42 | 143 ± 44 | 0.09 |
| Approach (transfemoral) | 245 (98) | 42 (100) | 203 (97) | 0.26 |
| Valve type (CoreValve) | 213 (85) | 33 (78) | 180 (86) | 0.2 |
| Valve size | ||||
| 23–26 mm | 155 (62) | 25 (59) | 130 (62) | 0.74 |
| 29–31 mm | 96 (38) | 17 (40) | 79 (38) | |
∗ Defined as patients with previous diagnosis by medical record or current statin therapy.
† Calculated by Modification of Diet in Renal Disease equation.
Echocardiographic variables of the patients included a mean aortic valve area of 0.6 ± 0.19 cm², with a peak and mean gradient of 77 ± 23 and 47.3 ± 15.1 mm Hg, respectively.
Mean serum creatinine level was 1.2 ± 0.4 mmol/L at the day before the procedure and was reduced significantly after 12 hours of hydration to a mean of 1 ± 0.2 mmol/L at the morning of the procedure (p <0.001). Mean preprocedural estimated glomerular filtration rate was 62.2 ± 18 ml/min/1.73 m 2 (calculated by Modification of Diet in Renal Disease equation).
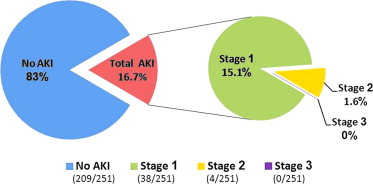
Forty-two patients (16.7%) developed AKI according to the VARC-2 updated criteria. AKI stages 1, 2, and 3 were diagnosed in 38 (15.1%), 4 (1.6%), and 0 patients (0%), respectively. No patient required RRT. The distribution of different AKI stages is illustrated in Figure 1 .
Clinical data, echocardiographic parameters, and procedural-related variables that were identified as predictors of increased risk of AKI after TAVI are CKD (OR 2.9, 95% CI 1.4 to 5.38, p = 0.002), PVD (OR 2.6, 95% CI 1.1 to 6.6, p = 0.045), higher EuroSCORE (p = 0.04), and blood transfusion (OR 2, 95% CI 1 to 3.9, p = 0.038). There was no association between hypertension, DM, previous MI, CHF, echocardiographic parameters, and contrast medium volume and the incidence of AKI ( Table 3 ). After performing multivariate logistic regression, baseline reduced creatinine clearance emerged as an independent predictor for TAVI-related AKI (OR 0.9, 95% CI 0.93 to 0.97 p <0.001).



