The no reflow phenomenon is reported to occur in >2% of all percutaneous coronary interventions (PCIs) and portends a poor prognosis. We analyzed data from 5,286 consecutive patients who underwent PCI from the Melbourne Interventional Group (MIG) registry from April 2004 through January 2008 who had 30-day follow-up completed. Patients without no reflow (normal reflow, n = 5,031) were compared to 255 (4.8%) with no reflow (n = 217 for transient no reflow, n = 38 for persistent no reflow). Patients with transient or persistent no reflow were more likely to present with ST-elevation myocardial infarction (MI) or cardiogenic shock (p <0.0001 for the 2 comparisons). They were also more likely to have complex lesions (American College of Cardiology/American Heart Association type B2/C), have lesions within a bypass graft, require an intra-aortic balloon pump, receive glycoprotein IIb/IIIa inhibition, and have a longer mean stent length (p <0.0001 for all comparisons). In-hospital outcomes were significantly worse in those patients with transient or persistent no reflow, with increased death, periprocedural MI, renal impairment, and major adverse cardiac events (p <0.0001 for all comparisons). Similarly, transient and persistent no reflow portended worse 30-day clinical outcomes, with a progressive increase in mortality (normal reflow 1.7% vs transient no reflow 5.5% vs persistent no reflow 13.2%, p <0.0001), MI, target vessel revascularization, and major adverse cardiac events (p <0.0001 for all comparisons) compared to patients with normal flow. In conclusion, transient or persistent no reflow complicates approximately 1 in 20 PCIs and results in stepwise increases in in-hospital and 30-day adverse outcomes.
Clinical predictors and the poor outcomes of patients with persistent no reflow have been well described, particularly in the setting of ST-segment elevation myocardial infarction (STEMI). However, the clinical implication of transient or reversible no reflow and no reflow in other clinical settings is not well characterized. Given the paucity of data on transient no reflow and because many previous studies were undertaken before the widespread use of potent antiplatelet agents and distal protection devices, we analyzed our large multicenter percutaneous coronary intervention (PCI) registry to determine clinical and procedural predictors of no reflow, which may provide insights into risk prediction of no reflow and therapeutic options. In addition, we compared 30-day clinical outcomes in unselected patients undergoing PCI who had normal myocardial reflow to those in patients with transient or persistent no reflow during the PCI procedure.
Methods
Data from consecutive patients enrolled in the Melbourne Interventional Group (MIG) registry undergoing PCI at each participating hospital site from April 2004 through January 2008 who had 30-day follow-up completed were analyzed. Of the 5,286 patients, 5,031 patients were classified as having normal anterograde coronary flow at the end of the PCI procedure, whereas 255 (4.8%) were assessed by the treating interventional cardiologist as having no reflow during or at the end of the PCI procedure (n = 217 for transient no reflow, 4.1%; n = 38 for persistent no reflow, 0.7%).
The MIG registry is a collaborative venture of interventional cardiologists practicing at 7 Australian tertiary referral hospitals that was designed to record data pertaining to all PCI procedures and to perform follow-up at 30 days and periodically thereafter. The MIG registry has been previously described in detail. Briefly, demographic, clinical, and procedural characteristics of consecutive patients undergoing PCI are prospectively recorded on case-report forms using standardized definitions for all fields. The registry is coordinated by the Centre of Cardiovascular Research and Education in Therapeutics, an independent research body within the School of Public Health and Preventive Medicine, Monash University (Melbourne, Australia). A data audit of 15 verifiable fields from 5% of randomly selected procedures at each institution is performed periodically by an investigator not affiliated with the institution; data accuracy has been 97%, which compares favorably to other large registries. The registry has been approved by the ethics committee in each participating hospital. “Opt-out” informed consent was obtained in all patients, as previously described.
Among other clinical variables, the indication for PCI was recorded and included patients with stable angina and those with unstable angina (with no increase in blood biomarkers), non-STEMI, and STEMI. Interventional strategy, stent selection, and antithrombotic therapy were left to the discretion of the operator in all procedures. Dual antiplatelet therapy was recommended in all cases where a stent was deployed.
PCI status was defined as elective, urgent, or rescue depending on the clinical indication. Elective PCIs were performed in all stable patients who presented electively for their procedure, often as outpatients. Urgent PCIs refer to all PCIs performed in inpatients in the context of acute coronary syndrome (ACS). Rescue PCI is defined as PCI performed for failed thrombolysis.
Normal anterograde coronary angiographic flow was defined as Thrombolysis In Myocardial Infarction (TIMI) grade 3 flow according to the standard definition developed by the TIMI study group. Persistent no reflow was defined as a TIMI grade ≤2 flow without residual epicardial coronary stenosis, dissection, or spasm. Transient no reflow was defined as a temporary decrease in flow (TIMI grade ≤2 flow) distal to the treated epicardial vessel segment, which resolved at the completion of the PCI procedure.
In-hospital complications were recorded at the time of discharge or death. Thirty-day follow up was conducted by telephone, and patient medical records were reviewed to verify events. All cardiac events were documented, including death (all-cause mortality), MI, target lesion revascularization, target vessel revascularization, and the composite of major adverse cardiac events (MACEs; consisting of death, MI, and/or target vessel revascularization). MI was defined as (1) an increase in creatine kinase or creatine kinase-MB ≥3 times the upper limit of normal and/or (2) a significant ST-segment change, development of new Q waves in ≥2 contiguous electrocardiographic leads, or new left branch bundle block pattern.
Acute renal failure was defined by an increase of serum creatinine to >200 μmol/L (2.27 mg/dl, or 2 times the baseline creatinine level) or need for dialysis. Stroke was defined by the sudden onset of persistent loss of neurologic function caused by an ischemic or hemorrhagic event during or after PCI. Cardiogenic shock was defined by hypotension (systolic blood pressure <90 mm Hg for ≥30 minutes or needing supportive measures), evidence of end-organ hypoperfusion or a cardiac index <2.2 L/min/m 2 , and a pulmonary capillary wedge pressure ≥15 mm Hg.
Continuous variables are expressed as mean ± SD, and categorical data are expressed as numbers/percentages. Continuous variables were compared using Student’s t test. Categorical variables were compared using Fisher’s exact or chi-square tests as appropriate. Independent predictors for transient and persistent no reflow and clinical outcomes were determined using multiple logistic regression models for variables with a p value <0.10 in simple logistic regression models. All calculated p values were 2 sided, and a p value <0.05 was considered statistically significant. Statistical analysis was performed using SPSS 12.0 (SPSS, Inc., Chicago, Illinois), for Windows (Microsoft, Redmond, Washington).
Results
Baseline characteristics of the cohort are presented in Table 1 . The incidence of transient or persistent no reflow in our cohort was 4.8% (n = 255). Patients with transient or persistent no reflow had higher baseline serum creatinine and lower left ventricular ejection fraction (p <0.05 for all comparisons). Fewer patients with no reflow compared to patients with normal reflow had diabetes, hypertension, dyslipidemia, and previous PCI (p <0.05 for all comparisons). When a 3-way comparison of patients with normal, transient, and persistent no reflow was performed (data not shown), left ventricular ejection fraction, hypertension, dyslipidemia, and previous PCI remained significantly different among groups (p <0.05).
| Variable | Normal Reflow (n = 5,031) | Transient or Persistent No Reflow (n = 255) | p Value |
|---|---|---|---|
| Age (years) | 64.6 ± 11.9 | 64.7 ± 12.9 | 0.82 |
| Men | 3,753 (74.6%) | 199 (78%) | 0.12 |
| Diabetes mellitus | 1,227 (24.4%) | 48 (18.8%) | 0.04 |
| Renal failure ⁎ | 190 (3.8%) | 8 (3.1%) | 0.74 |
| Serum creatinine (μmol/L) | 100 ± 80 | 110 ± 180 | 0.04 |
| Hypertension † | 3,259 (64.9%) | 140 (54.9%) | 0.002 |
| Dyslipidemia ‡ | 3,581 (71.6%) | 154 (61.6%) | 0.001 |
| Current smoker | 1,120 (22.5%) | 50 (20%) | 0.39 |
| Previous myocardial infarction | 1,548 (30.8%) | 75 (29.4%) | 0.68 |
| Previous percutaneous coronary intervention | 1,244 (24.7%) | 45 (17.7%) | 0.01 |
| Previous coronary artery bypass graft surgery | 472 (9.4%) | 31 (12.2%) | 0.15 |
| History of heart failure | 185 (3.7%) | 10 (3.9%) | 0.86 |
| Left ventricular ejection fraction (%) | 55.1 ± 12.0 | 51.2 ± 12.6 | 0.001 |
| Cerebrovascular disease | 300 (6.0%) | 16 (6.3%) | 0.79 |
| Peripheral arterial disease | 343 (6.8%) | 23 (9.1%) | 0.16 |
⁎ Serum creatinine level >200 μmol/L.
† History of hypertension diagnosed and treated with medication, systolic blood pressure >140 mm Hg or diastolic blood pressure >90 mm Hg diastolic on ≥2 occasions, or current use of antihypertensive medication.
‡ History of dyslipidemia diagnosed and/or treated with medication or fasting total cholesterol level >5.0 mmol/L, high-density lipoprotein level <1.0 mmol/L, or triglyceride level >2.0 mmol/L.
Clinical and procedural characteristics of the 2 patient groups are presented in Table 2 . Patients with no reflow were more likely to present with an ACS, STEMI, or cardiogenic shock than their normal reflow counterparts (p <0.0001 for all comparisons). In patients with stable angina, unstable angina, non-STEMI, and STEMI, incidences of no reflow were 2.2%, 3.3%, 5.7%, and 8.7% respectively, with significant differences observed only for no reflow versus normal flow in patients with stable angina and STEMI (p <0.0001). Symptom-to-door time in patients with STEMI was longer in patients with no reflow than with normal reflow (213.5 ± 282.6 vs 167.3 ± 190.1 minutes, p = 0.06).
| Variable | Normal Reflow | Transient or Persistent No Reflow | p Value |
|---|---|---|---|
| (n = 5,031) | (n = 255) | ||
| Stable angina | 1,728 (34.5%) | 38 (14.9%) | <0.0001 |
| Acute coronary syndrome | 2,980 (59.2%) | 210 (82.4%) | <0.0001 |
| Unstable angina | 526 (10.5%) | 18 (7.1%) | 0.09 |
| Non–ST-segment elevation myocardial infarction | 1,207 (24.1%) | 73 (28.6%) | 0.17 |
| ST-segment elevation myocardial infarction | 1,247 (24.9%) | 119 (46.7%) | <0.0001 |
| Symptoms to door (minutes) | 167.3 ± 190.1 | 213.5 ± 282.6 | 0.06 |
| Door to balloon (minutes) | 132.1 ± 152.4 | 144.9 ± 194.0 | 0.47 |
| Killip class I | 2,618 (89.7%) | 165 (82.5%) | 0.003 |
| Cardiogenic shock | 104 (2.1%) | 18 (7.1%) | <0.0001 |
| Thrombolysis | 277 (5.5%) | 19 (7.5%) | 0.20 |
| Glycoprotein IIb/IIIa inhibitor | 1,339 (26.6%) | 158 (62%) | <0.0001 |
| Unfractionated or low-molecular-weight heparin before percutaneous coronary intervention | 1,862 (37%) | 127 (49.8%) | <0.0001 |
| Clopidogrel preload | 2,384 (47.4%) | 102 (40.0%) | 0.02 |
| Mean stent length (mm) | 16.60 ± 5.36 | 18.37 ± 5.26 | <0.0001 |
| Mean stent diameter (mm) | 2.92 ± 0.47 | 3.03 ± 0.51 | <0.0001 |
| Vessel diameter stenosis before percutaneous coronary intervention culprit (%) | 86.76 ± 11.50 | 91.63 ± 10.62 | <0.0001 |
| Thrombolysis In Myocardial Infarction grade 3 flow before percutaneous coronary intervention | 4,249 (70.6%) | 116 (38.4%) | <0.0001 |
| Lesion characteristics | |||
| American College of Cardiology/American Heart Association type B2/C lesion | 2,951 (48.4%) | 223 (72.6%) | <0.0001 |
| Ostial lesions | 248 (4.1%) | 17 (5.5%) | 0.24 |
| Bifurcations | 427 (7.0%) | 32 (10.4%) | 0.03 |
| Long-term total occlusions | 240 (3.9%) | 16 (5.2%) | 0.29 |
| Multivessel disease | 3,687 (60.6%) | 193 (62.9%) | 0.47 |
| De novo lesion | 5,734 (94%) | 296 (96.4%) | 0.08 |
| Bypass graft | 179 (2.9%) | 25 (8.1%) | <0.0001 |
| Restenosis lesions | 355 (5.8%) | 11 (3.6%) | 0.10 |
| Stent deployed | 5,587 (91.6%) | 261 (85%) | <0.0001 |
| Intra-aortic balloon pump | 96 (1.9%) | 16 (6.3%) | <0.0001 |
| Distal embolic protection device | 82 (1.3%) | 13 (4.2%) | 0.001 |
Patients with no reflow were also more likely to have undergone bifurcation PCI, PCI of more complex lesions (American College of Cardiology/American Heart Association B2/C lesions), or PCI of bypass grafts. In addition, patients with no reflow had been treated with longer mean stent lengths, had greater diameter lesion stenosis severity before PCI, and less TIMI grade 3 flow in the culprit vessel before PCI. Higher preprocedural usage of unfractionated heparin or low-molecular-weight heparin, intraprocedural usage of glycoprotein IIb/IIIa inhibitors, and intra-aortic balloon pump and distal embolic protection devices were observed in patients with no reflow, although fewer had clopidogrel preloading. More patients with normal reflow had successful stent deployment.
Significant multivariate predictors of no reflow are presented in Table 3 and include the use of glycoprotein IIb/IIIa inhibitors, bypass-graft PCI, and more complex culprit lesions. Successful stent deployment was the only protective independent predictor against no reflow.
| Variable | OR | 95% CI | p Value |
|---|---|---|---|
| Glycoprotein IIb/IIIa inhibitor | 3.02 | 2.20–4.12 | <0.0001 |
| Bypass-graft percutaneous coronary intervention | 2.51 | 1.23–5.14 | 0.01 |
| American College of Cardiology/American Heart Association type B2/C lesion | 1.74 | 1.28–2.35 | <0.0001 |
| Stent deployment | 0.48 | 0.30–0.78 | 0.003 |
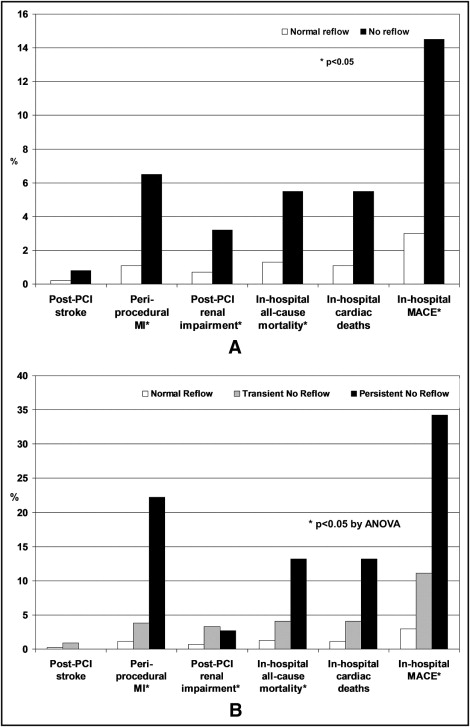
Postprocedure and in-hospital complications and 30-day clinical outcomes are presented in Tables 4 and 5 and Figures 1 and 2 , respectively. Compared to patients with normal reflow, more patients with no reflow developed periprocedural MI, renal impairment, and in-hospital deaths (p ≤0.001 for all comparisons). Analysis of outcomes according to whether no reflow was transient or persistent revealed a stepwise increase in periprocedural and in-hospital complications in patients with normal reflow, transient no reflow, and persistent no reflow, respectively, after PCI ( Figure 1 , Table 4 ).
| Variable | Normal Reflow Versus No Reflow | Normal Reflow Versus Transient No Reflow Versus Persistent No Reflow | |||||
|---|---|---|---|---|---|---|---|
| Normal Reflow | Transient or Persistent No Reflow | p Value | Normal Reflow | Transient No Reflow | Persistent No Reflow | p Value | |
| (n = 5,031) | (n = 255) | (n = 5,031) | (n = 217) | (n = 38) | |||
| Postprocedure stroke | 9 (0.2%) | 2 (0.8%) | 0.10 | 9 (0.2%) | 2 (0.9%) | 0 (0%) | 0.06 |
| Periprocedural myocardial infarction | 55 (1.1%) | 16 (6.5%) | <0.0001 | 55 (1.1%) | 8 (3.8%) | 8 (22.2%) | <0.0001 |
| Renal impairment after percutaneous coronary intervention | 36 (0.7%) | 8 (3.2%) | 0.001 | 36 (0.7%) | 7 (3.3%) | 1 (2.7%) | <0.0001 |
| In-hospital all-cause mortality | 67 (1.3%) | 14 (5.5%) | <0.0001 | 67 (1.3%) | 9 (4.1%) | 5 (13.2%) | <0.0001 |
| In-hospital cardiac deaths | 57 (1.1%) | 14 (5.5%) | <0.0001 | 57 (1.1%) | 9 (4.1%) | 5 (13.2%) | <0.0001 |
| In-hospital major adverse cardiac events | 153 (3%) | 37 (14.5%) | <0.0001 | 153 (3%) | 24 (11.1%) | 13 (34.2%) | <0.0001 |
| Variable | Normal Reflow Versus No Reflow | Normal Reflow Versus Transient Versus Persistent No Reflow | |||||
|---|---|---|---|---|---|---|---|
| Normal Reflow | Transient or Persistent No Reflow | p Value | Normal Reflow | Transient No Reflow | Persistent No Reflow | p Value | |
| (n = 5,031) | (n = 255) | (n = 5,031) | (n = 217) | (n = 38) | |||
| All-cause mortality | 85 (1.7%) | 17 (6.7%) | <0.0001 | 85 (1.7%) | 12 (5.5%) | 5 (13.2%) | <0.0001 |
| Cardiac death (percent all deaths) | 70 (1.4%) | 17 (6.7%) | <0.0001 | 70 (1.4%) | 12 (5.5%) | 5 (13.2%) | <0.0001 |
| Myocardial infarction | 108 (2.1%) | 17 (6.7%) | <0.0001 | 108 (2.1%) | 9 (4.1%) | 8 (21.1%) | <0.0001 |
| Target lesion revascularization | 79 (1.6%) | 13 (5.1%) | <0.0001 | 79 (1.6%) | 9 (4.1%) | 4 (10.5%) | <0.0001 |
| Target vessel revascularization | 96 (1.9%) | 15 (5.9%) | <0.0001 | 96 (1.9%) | 10 (4.6%) | 5 (13.2%) | <0.0001 |
| 30-day major adverse cardiac events | 239 (4.8%) | 42 (16.5%) | <0.0001 | 239 (4.8%) | 28 (12.9%) | 14 (36.8%) | <0.0001 |