The aim of this study was to evaluate the prognostic value of brain natriuretic peptide (BNP) in outpatients with the Eisenmenger syndrome (ES). BNP is often elevated in patients with cyanotic congenital heart disease. The clinical utility of BNP in patients with cyanotic congenital heart disease and the ES has not been clearly delineated. Records of adults with ES who had undergone serum BNP measurement were reviewed. The primary end point was death or heart failure admission. Fifty-three patients were included, with 15 patients (28%) meeting the primary end point (death in 7, heart failure hospitalization in 8). Mean and median baseline BNP in patients meeting the primary end point were 322 ± 346 and 179 pg/ml, compared to 100 ± 157 and 41 pg/ml in those not meeting the primary end point (p = 0.0029). A Cox proportional-hazards model using baseline BNP between the 2 groups yielded a hazard ratio of 1.84 (95% confidence interval [CI] 1.19 to 2.85, p = 0.006). The relative risk for baseline BNP level >140 pg/ml was 4.62 (95% CI 1.80 to 11.3, p = 0.008). Patients who met the primary end point increased their BNP levels by 42.5 pg/ml per year (95% CI 12.09 to 72.95, p = 0.006) compared to 7.2 pg/ml per year (95% CI 2.01 to 12.47, p = 0.007) in patients who did not meet the primary end point. In conclusion, elevated BNP levels are predictive of death or heart failure admission in patients with the ES. A serum BNP level >140 pg/ml is a useful tool in identifying high-risk patients.
Brain natriuretic peptide (BNP) is a powerful prognostic marker for heart failure (HF) and death in a variety of cardiopulmonary conditions, including coronary artery disease and isolated pulmonary hypertension. Studies have shown that BNP levels are increased to varying extents in most types of repaired and unrepaired congenital heart disease. Researchers have attempted to validate the use of BNP in a wide range of congenital heart diseases as well as correlate BNP with exercise capacity and echocardiographic measurement of performance. There is a wide range of BNP values obtained from patients with the Eisenmenger syndrome (ES) that reflects the heterogenous nature of this population. Trojnarska et al reported an average BNP of 76 pg/ml (range 14 to 344) in 24 patients with the ES. Those with increased BNP levels were found to have lower exercise capacity. The objective of this study was to assess the prognostic value of serum BNP in adults with the ES.
Methods
After institutional review board approval was obtained, medical records of patients with the ES followed at the Ahmanson/UCLA Adult Congenital Heart Disease Center from January 2003 to January 2010 were reviewed. The ES was defined as a peripheral oxygen saturation of <90%, unrepaired intra- or extracardiac shunt, and severe pulmonary hypertension by either echocardiography or cardiac catheterization. Patients were classified as having simple or complex lesions. Simple anatomy was defined as atrial septal defect, ventricular septal defect, or patent ductus arteriosus. Complex anatomy included atrioventricular septal defects, tetralogy of Fallot with shunt physiology or pulmonary atresia, single-ventricle physiology, d-transposition of the great arteries, and truncus arteriosus.
Patients who had single consultations or who did not have serum BNP levels obtained in the outpatient setting were excluded from the analysis. All outpatient serum BNP measurements, 6-minute walk distances, cardiopulmonary exercise test results, echocardiographic parameters, and catheterization data were compiled. All patients included in the study had serum BNP levels measured within the first 3 outpatient visits. All BNP measurements were obtained using the Biosite assay (Alere, Inc., Waltham, Massachusetts) by the UCLA clinical laboratory, with a reference range of <100 pg/ml.
The primary end point was death or hospitalization for HF. Hospitalization for HF was defined as volume overload (elevated jugular venous pressure, increased peripheral edema, ascites, and/or pulmonary edema) requiring hospitalization and intravenous diuretic administration. Two-tailed unpaired Student’s t tests, Cox proportional-hazard models, and a linear regression model using a generalized least squares transformation were used to compare the 2 groups. Statistical analysis was performed using Stata version 11.0 (StataCorp LP, College Station, Texas). A p value <0.05 was considered statistically significant in all analyses. Natural log transformation of serum BNP values was used to normalize the data for the Cox proportional-hazard models. The Cox proportional-hazards regression is expressed as hazard ratios with 95% confidence intervals [CIs].
Results
A total of 67 patients were identified. Fourteen were excluded from analysis: 12 patients did not have BNP measurements in the outpatient setting and 2 had single consultations and were subsequently followed elsewhere; 53 patients met the inclusion criteria.
Baseline demographic data are listed in Table 1 . The average age of the patient population at baseline was 44 ± 11.2 years, and 39% of the patients were men. The mean room oxygen saturation at rest was 75 ± 7.9%. Most patients (62%) had complex anatomy. The median follow-up time was 1,152 days. Baseline pharmacologic therapies included β blockers in 13 patients, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers in 10, digoxin in 7, and diuretics in 17. Additionally, 23 patients were receiving oral pulmonary vasodilator therapy consisting of sildenafil and/or bosentan.
| Variable | Patients | HF Hospitalization | Death | HF Hospitalization or Death |
|---|---|---|---|---|
| (n = 53) | (n = 8 [15%]) | (n = 7 [13%]) | (n = 15 [28%]) | |
| Age (years) | 44 ± 11.2 | 37 ± 11 | 44 ± 11 | 40 ± 11 |
| Men | 21 (39%) | 3 (37%) | 0 | 3 (20%) |
| Room air saturation at rest | 75 ± 7.9% | 74 ± 8% | 73 ± 7% | 73 ± 7% |
| Atrial septal defect | 2 | 1 | 1 | 2 |
| Ventricular septal defect | 15 | 2 | 2 | 4 |
| Patent ductus arteriosus | 4 | 0 | 0 | 0 |
| Single ventricle | 15 | 3 | 1 | 4 |
| Atrioventricular septal defect | 6 | 1 | 2 ⁎ | 3 |
| D-transposition of the great arteries with ventricular septal defect | 3 | 0 | 1 | 1 |
| Truncus arteriosus | 4 | 1 | 0 | 1 |
| Tetralogy of Fallot with shunt or tetralogy of Fallot/pulmonary atresia | 4 | 0 | 0 | 0 |
⁎ The 2 patients who died with atrioventricular septal defects also had a patent ductus arteriosus.
The primary end point ( Table 1 ), defined as death or HF hospitalization, occurred in 15 patients (28%) at a mean of 783 ± 440 days. Seven patients (13%) died, and records demonstrated that most of these patients were experiencing progressive symptoms of dyspnea, with examination findings suggestive of right heart failure (edema, elevated jugular venous pressure). Eight patients (15%) were hospitalized for congestive HF, 4 of whom had associated arrhythmias during their hospitalizations (1 atrial fibrillation, 1 atrioventricular nodal reentrant tachycardia, and 2 ventricular tachycardia). Two patients were hospitalized for worsening HF symptoms and subsequently underwent heart-lung transplantation.
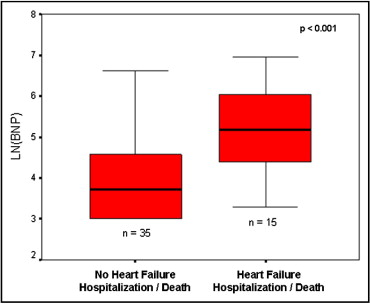
Patients who met the primary end point had higher mean and median baseline BNP levels of 322 ± 346 and 179 pg/ml, compared to 100 ± 157 and 41 pg/ml (p = 0.0029) in those who did not ( Figure 1 , Tables 2 and 3 ). Patients who met the primary end point did so at a mean of 783 ± 440 days, or just over 2 years from their first outpatient serum BNP measurement. A Cox proportional-hazards model between the 2 groups yielded a hazard ratio of 1.84 (95% CI 1.19 to 2.85, p = 0.006).
| Variable | No Congestive HF Hospitalization or Death | Congestive HF Hospitalization or Death | Mean Follow-Up (days) | Mean Follow-Up Before End Point (days) | p Value |
|---|---|---|---|---|---|
| (n = 38) | (n = 15) | ||||
| Baseline outpatient BNP (pg/ml) | 100 ± 157 | 322 ± 346 | 994 ± 625 | 783 ± 440 | 0.0029 |
| Maximum outpatient BNP (pg/ml) | 130 ± 177 | 674 ± 899 | 725 ± 602 | 433 ± 389 | 0.0015 |
| 6-minute walk distance (m) | 297 ± 112 (n = 26) | 213 ± 95 (n = 9) | 774 ± 496 | 775 ± 440 | 0.0726 |
| Maximum oxygen saturation (ml/kg/min) | 12.03 ± 3.1 (n = 16) | 11.1 ± 3.44 (n = 8) | 1,045 ± 396 | 667 ± 420 | 0.5561 |
| Left ventricular or single-ventricle EF (%) (n = 47) | 54 ± 9 | 51 ± 10 | 1,029 ± 618 | 715 ± 402 | 0.3508 |
| Right ventricular or single ventricle EF (%) (n = 36) | 44 ± 9 | 38 ± 13 | 982 ± 608 | 715 ± 402 | 0.1303 |
| Diagnosis | BNP (pg/ml) | p Value | |
|---|---|---|---|
| End Point Not Met | End Point Met | ||
| Simple | 92 (20–341) | 535 (54–1,000) | 0.0015 |
| Atrial septal defect | 0 | 875 (751–1,000) | |
| Ventricular septal defect | 73 (20–247) | 365 (54–1,050) | |
| Patent ductus arteriosus | 143 (41–341) | 0 | |
| Complex | 112 (20–745) | 145 (27–506) | 0.6434 |
| Single ventricle | 102 (20–745) | 100 (27–179) | |
| Atrioventricular septal defect | 228 (20–611) | 124 (76–187) | |
| D-transposition of the great arteries | 36 (20–52) | 506 | |
| Truncus arteriosus | 93 (28–210) | 27 | |
| Tetralogy of Fallot with shunt or tetralogy of Fallot/pulmonary atresia | 107 (59–174) | 0 | |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


