Chronic kidney disease increases cardiovascular risk and all-cause mortality. However, data on the predictive power of dynamic changes in kidney function are sparse. The aim of this research was to assess the predictive power of serial changes in kidney function on mortality and cardiovascular risk. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation at baseline and at follow-up in a high-risk population of 619 consecutive patients who underwent coronary angiography. The population was stratified into 3 groups with respect to decreases in eGFR: stable kidney function (no decrease in eGFR) versus a mild decline (decrease in eGFR >0 but <4 ml/min/1.73 m 2 per year) and a rapid decline in kidney function (decrease in eGFR ≥4 ml/min/1.73 m 2 per year). Mortality and nonfatal cardiovascular events were recorded over 4 years. Baseline coronary angiography revealed significant coronary stenoses (≥50%) in 368 patients (60%). Survival and event-free survival were significantly lower in patients with rapid decreases in eGFR compared with those with mild decreases (p <0.001 and p = 0.012, respectively) and stable kidney function (p <0.001 and p = 0.004, respectively). After multivariate adjustment in Cox regression analyses, the continuous variable decline in kidney function significantly predicted death (standardized adjusted hazard ratio 1.32, 95% confidence interval 1.03 to 1.70, p = 0.032) and the incidence of the composite end point death and nonfatal vascular events (hazard ratio 1.20, 95% confidence interval 1.01 to 1.43, p = 0.038). A 5 ml/min/1.73 m 2 decrease in eGFR independently conferred a 60% increase in mortality risk (p = 0.032). In conclusion, a rapid decline in kidney function is a powerful and independent new risk marker for death and vascular events.
Decreased estimated glomerular filtration rate (eGFR) not only increases cardiovascular risk but also is associated with all-cause mortality. All-cause mortality is the highest level in the hierarchical ranking of outcome information to be considered in the analysis of clinical trial data. To clarify the prognostic role of a dynamic decline in kidney function, we ascertained the longitudinal course of eGFR in a high-risk cohort of coronary patients by doing a serial 2-point measurement of eGFR. Afterward, we collected clinical end points over the subsequent 4 years in this high-risk population of coronary patients who underwent coronary angiography.
Methods
From 2009 to 2010, we enrolled a consecutive cohort of 625 white patients who were referred for coronary angiography for the evaluation of established or suspected stable coronary artery disease (CAD). Three patients with end-stage renal disease at baseline and 3 patients with type 1 diabetes were excluded. In the present analysis, we therefore included 619 patients for whom baseline and follow-up serum creatinine values were available. Patients biannually underwent follow-up visits at our institution. Follow-up serum creatinine was measured at the 4-year follow-up visit. If a proband did not attend the 4-year follow-up visit (n = 184), serum creatinine measured at 2-year follow-up was used for eGFR calculation. Therefore, creatinine was measured solely during elective follow-up visits. The mean time from baseline to the second ascertainment of serum creatinine was 3.5 ± 0.8 years.
At baseline and at follow-up, height and weight were recorded, and information on conventional cardiovascular risk factors (history of smoking, hypertension, established diabetes mellitus, and family history of atherosclerotic disease) was obtained by a standardized interview. Systolic and diastolic blood pressure was measured using the Riva-Rocci method under rest conditions in a sitting position on the day of hospital entry. Diabetes mellitus was diagnosed according to World Health Organization criteria, and hypertension was defined according to the 2013 European Society of Cardiology and European Society of Hypertension guidelines. Body mass index was calculated as body weight in kilograms divided by the square of height in meters.
Of our patients, 66% were taking aspirin, 43% statins, 3% fibrates, 36% angiotensin-converting enzyme inhibitors, and 4% angiotensin II–blocking agents. Among 133 patients with diabetes, 43% were not receiving any antidiabetic medications, and 35%, 32%, and 23% were receiving (alone or in combination) sulfonylurea, biguanides, and insulin, respectively. Before inclusion in the study, 185 patients (30%) had experienced myocardial infarctions, 16 (3%) strokes, 76 (12%) percutaneous coronary intervention, and 39 (6%) coronary artery bypass grafting. At baseline, coronary angiography was performed using the Judkins technique. Coronary stenoses with luminal narrowing ≥50% were considered significant, and patients with any visible arterial lesions were recorded as having CAD, as described previously. The present study complies with the Declaration of Helsinki, the ethics committee of the University of Innsbruck approved the present study, and all participants gave written informed consent.
The serum levels of triglycerides, total cholesterol, and low-density lipoprotein cholesterol were determined using enzymatic hydrolysis and precipitation techniques (Triglycerides GPO-PAP, CHOD/PAP, QuantolipLDL, QuantolipHDL; Roche Diagnostics GmbH, Basel, Switzerland) on a Hitachi Analyzer 717 or 911 (Hitachi, Tokyo, Japan). Creatinine was measured using a kinetic Jaffe method (Roche Diagnostics GmbH) using a Hitachi 717 or 911 or a Cobas Integra 800 (Roche Diagnostics GmbH). Because the Modification of Diet in Renal Disease (MDRD) eGFR was evaluated in patients with kidney disease and tends to underestimate glomerular filtration rate in patients with rates >60 ml/min/1.73 m 2 , eGFR was assessed using the Chronic Kidney Disease Epidemiology Collaboration equation, which in patients with nearly normal renal function has been shown to give more accurate estimates of GFR than the MDRD equation.
During an average follow-up period of 4.1 ± 1.2 years beginning right after the second ascertainment of serum creatinine, we recorded total mortality and the composite end point of major cardiovascular events, including death, nonfatal myocardial infarction, nonfatal ischemic stroke, need for coronary artery bypass grafting and percutaneous coronary intervention, and need for noncoronary revascularizations. All recorded end points occurred after the second measurement of creatinine. Follow-up data were available for all 619 patients. Patients biannually underwent follow-up visits at our institution, when information on cardiovascular end points was obtained in a standardized interview. Study end points reported by the patients were cross-checked against medical records. Furthermore, follow-up data were collected by telephone contacts with patients and family physicians and through a national registry of death.
Differences in baseline characteristics were tested for statistical significance using chi-square and the Mann-Whitney U tests for categorical and continuous variables, respectively. Adjusted hazard ratios (HRs) for the incidence of vascular events were derived from Cox proportional-hazards models; for these calculations, continuous variables were z-transformed.
With respect to kidney function, we provide standardized HRs, which refer to the risk increase conferred by a 1-SD increase in a continuous variable. The SD of the decrease in eGFR per year was 3.0 ml/min/1.73 m 2 . Our risk estimates therefore refer to a 3.0 ml/min/1.73 m 2 increase in the decrease of eGFR, respectively (a greater decrease is associated with increased risk, so HRs here are >1). Furthermore, we compared risk in groups with respect to the decrease in eGFR: stable kidney function (decrease in eGFR ≤0 ml/min/1.73m 2 per year) versus a mild decline in kidney function (decrease in eGFR >0 but <4 ml/min/1.73m 2 per year) and a rapid decline in kidney function (decrease in eGFR ≥4 ml/min/1.73 m 2 per year). Overall, the final model adjusted for age, gender, baseline eGFR, type 2 diabetes, smoking, body mass index, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, blood pressure, high-sensitivity C-reactive protein, the presence of significant stenoses at baseline coronary angiography, incidence of diabetes or vascular events (nonfatal myocardial infarction, nonfatal ischemic stroke, need for coronary artery bypass grafting and percutaneous coronary intervention, or need for noncoronary revascularizations) during the period from baseline coronary angiography to the second measurement of serum creatinine, and use of statins and angiotensin-converting enzyme inhibitors or angiotensin II antagonists at baseline and at the second measurement of serum creatinine. Sample size calculations showed that assuming a mean survival of patients with stable kidney function of 4 years and a ratio of patients with stable kidney function to those with rapid decline in kidney function of 6:1, 56 patients would be needed per study group to detect a relative risk difference in survival of 40% with a power of 80% at an α level of 0.05. Results are expressed as mean ± SD if not denoted otherwise. All statistical analyses were performed using SPSS version 11.0 for Windows (SPSS, Inc., Chicago, Illinois).
Results
At baseline, 86% of our patients had eGFRs ≥60 ml/min/1.73 m 2 . Serial decreases in eGFR from baseline to the second ascertainment of renal function occurred in 260 (42%) patients; the mean eGFR at follow-up was 81 ± 17 ml/min/1.73 m 2 . In patients with stable kidney function, mean eGFR increased to 87 ± 14 ml/min/1.73m 2 (p <0.001), whereas mean eGFR decreased to 75 ± 16 ml/min/1.73 m 2 in patients with mild declines in kidney function (p <0.001) and to 53 ± 17 ml/min/1.73 m 2 in patients with rapid declines in kidney function (p <0.001). Table 1 lists the baseline characteristics of our patients with respect to the change in eGFR.
| Variable | Whole Study Cohort (n = 619) | Stable Kidney Function (n = 359) | Mild Decline in Kidney Function (n = 237) | Rapid Decline in Kidney Function (n = 23) | p Value |
|---|---|---|---|---|---|
| Age (yrs) | 62 ± 10 | 62 ± 10 | 64 ± 10 | 68 ± 9 | 0.002 |
| Significant stenoses ≥50% | 60% | 61% | 59% | 44% | 0.240 |
| Type 2 diabetes mellitus | 22% | 20% | 21% | 48% | 0.007 |
| Body mass index (kg/m 2 ) | 27.3 ± 4.0 | 27.3 ± 4.0 | 27.1 ± 3.8 | 28.5 ± 4.4 | 0.193 |
| Hypertension | 68% | 66% | 70% | 87% | 0.078 |
| Smokers | 57% | 58% | 59% | 35% | 0.084 |
| Serum creatinine (mg/dl) | 1.1 ± 0.2 | 1.1 ± 0.2 | 1.1 ± 0.2 | 1.0 ± 0.3 | 0.152 |
| eGFR (ml/min/1.73 m 2 ) | 78 ± 15 | 79 ± 15 | 79 ± 15 | 69 ± 16 | 0.012 |
| Total cholesterol (mg/dl) | 218 ± 43 | 221 ± 44 | 214 ± 43 | 217 ± 38 | 0.066 |
| Low-density lipoprotein (mg/dl) | 131 ± 35 | 132 ± 36 | 130 ± 35 | 131 ± 32 | 0.485 |
| High-density lipoprotein (mg/dl) | 49 ± 14 | 48 ± 14 | 51 ± 15 | 46 ± 13 | 0.829 |
| Triglycerides (mg/dl) | 164 ± 106 | 173 ± 115 | 145 ± 76 | 176 ± 130 | 0.133 |
| Fasting glucose (mg/dl) | 115 ± 35 | 112 ± 30 | 116 ± 38 | 124 ± 45 | 0.104 |
| Glycosylated hemoglobin (%) | 6.1 ± 1.0 | 6.0 ± 0.9 | 6.1 ± 1.0 | 6.6 ± 1.3 | 0.042 |
| Systolic blood pressure (mm Hg) | 135 ± 22 | 133 ± 22 | 137 ± 21 | 136 ± 19 | 0.240 |
| Diastolic blood pressure (mm Hg) | 79 ± 12 | 78 ± 12 | 79 ± 12 | 79 ± 11 | 0.618 |
| High-sensitivity C-reactive protein (mg/dl) | 0.62 ± 1.17 | 0.57 ± 0.64 | 0.65 ± 1.68 | 0.91 ± 1.45 | 0.148 |
| Angiotensin-converting enzyme inhibitors | 36% | 32% | 41% | 39% | 0.068 |
| Angiotensin II receptor blockers | 4% | 4% | 4% | 13% | 0.113 |
| Statins | 44% | 42% | 46% | 44% | 0.699 |
During the 4-year follow-up period after the second measurement of eGFR, we recorded 173 first clinical events representing the composite end point (67 deaths, 24 nonfatal myocardial infarctions, 28 nonfatal ischemic strokes, 8 coronary artery bypass graft procedures, 30 percutaneous coronary interventions, and 16 revascularizations at the carotid or peripheral arteries). In total, 81 patients died during the period of event assessment.
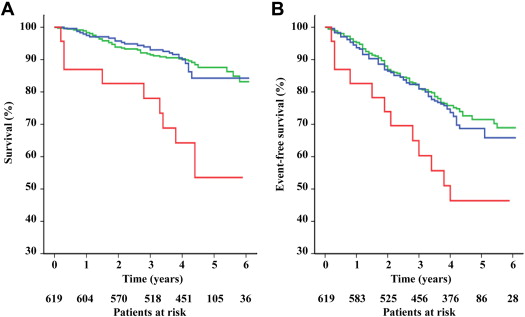
The mortality rate was 12% in patients with stable eGFRs compared with 13% in patients with mild and 39% in those with rapid declines in kidney function (p for trend = 0.001). Also, survival was significantly lower in patients with rapid declines compared with mild declines and stable kidney function (p <0.001 for both analyses), whereas survival did not differ significantly between stable and mildly declining kidney function (p = 0.579) ( Figure 1 ). When adjusted for baseline eGFR, age, gender, body mass index, blood pressure, type 2 diabetes, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, smoking, and high-sensitivity C-reactive protein in Cox regression analysis, the continuous variable decline in kidney function was significantly associated with total mortality over the 4 years between follow-up and the end of the study ( Table 2 ). This result was stable after further adjustment for the angiographic presence of significant stenoses, treatment with statins and angiotensin-converting enzyme inhibitors or angiotensin II–blocking agents at baseline and at follow-up, and the incidence of diabetes or vascular events during the period from baseline coronary angiography to the serial measurement of serum creatinine in the final model. An interaction term, baseline eGFR by decrease in eGFR, was not significant (p = 0.093), indicating that baseline eGFR did not significantly modulate the impact of the decrease in eGFR on the risk for death. A 5 ml/min/1.73 m 2 decrease in glomerular filtration rate conferred a 60% increased mortality risk in this fully adjusted model (HR 1.60, 95% confidence interval 1.04 to 2.44, p = 0.032).
| Model | All-Cause Mortality | Major Cardiovascular Events | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| All patients | ||||||
| Crude | 1.42 | 1.11–1.82 | 0.006 | 1.23 | 1.04–1.45 | 0.013 |
| Model 1 ∗ | 1.33 | 1.04–1.70 | 0.022 | 1.18 | 1.01–1.40 | 0.046 |
| Model 2 † | 1.32 | 1.03–1.70 | 0.032 | 1.20 | 1.01–1.43 | 0.038 |
| Patients with coronary artery disease | ||||||
| Crude | 1.42 | 1.07–1.89 | 0.016 | 1.21 | 1.01–1.45 | 0.038 |
| Model 1 ∗ | 1.42 | 1.07–1.87 | 0.014 | 1.22 | 1.02–1.46 | 0.029 |
| Model 2 † | 1.41 | 1.06–1.89 | 0.020 | 1.23 | 1.02–1.49 | 0.029 |
| Patients free of events during serial creatinine measurement | ||||||
| Crude | 1.46 | 1.12–1.92 | 0.006 | 1.25 | 1.05–1.50 | 0.015 |
| Model 1 ∗ | 1.36 | 1.03–1.79 | 0.030 | 1.21 | 1.01–1.46 | 0.047 |
| Model 2 † | 1.36 | 1.02–1.80 | 0.034 | 1.25 | 1.03–1.52 | 0.027 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


