Balloon predilation is historically considered a requirement before performing transcatheter aortic valve implantation (TAVI). As the procedure has evolved, it has been questioned whether it is actually needed, but data are lacking on mid-term outcomes. The aim of this study was to evaluate the effect of balloon predilation before TAVI. A total of 517 patients who underwent transfemoral TAVI from November 2007 to October 2015 were analyzed. The devices implanted included the Medtronic CoreValve (n = 216), Medtronic Evolut R (n = 30), Edwards SAPIEN XT (n = 210), and Edwards SAPIEN 3 (n = 61). Patients were divided into 2 groups depending on whether pre-implantation balloon aortic valvuloplasty (pre-BAV) was performed (n = 326) or not (n = 191). Major adverse cardiac and cerebrovascular events (MACCE) were primarily evaluated. Propensity score matching was used to adjust for differences in baseline characteristics and potential confounders (n = 113 pairs). In the overall cohort, patients without pre-BAV had a significantly higher MACCE rate at 30 days, driven by a higher incidence of stroke (0.3% pre-BAV vs 3.7% no-pre-BAV, p <0.01). MACCE and mortality at 1 year were, however, similar in both groups. Independent predictors of MACCE at 1 year included serum creatinine, NYHA class 3 to 4, logistic European System for Cardiac Operative Risk Evaluation, and postdilation. Of note, the postdilation rate was higher in the no-pre-BAV group (21.5% pre-BAV vs 35.6% no-pre-BAV, p <0.001). After propensity score matching, there were no differences in MACCE between the 2 groups. In conclusion, this study showed that, in selected patients and with specific transcatheter valves, TAVI without pre-BAV appears to be associated with similar mid-term outcomes compared with TAVI with pre-BAV, but it may increase the need for postdilation.
Transcatheter aortic valve implantation (TAVI) has rapidly become the treatment of choice for high risk and inoperable patients with severe symptomatic aortic stenosis, and randomized trials are ongoing in intermediate-risk patients. Historically, balloon aortic valvuloplasty (BAV) before device implantation is performed to ensure adequate apposition of the valve and to facilitate crossing of the valve and optimal expansion and is widely believed to be a necessary step before implanting transcatheter aortic valves. However, any extra step in a procedure may increase the risk of complications. BAV can cause stroke, conduction disturbances requiring permanent pacemaker implantation, and even annular rupture. In addition, it requires rapid pacing which must be used with caution in patients with poor left ventricular function. To date, only preliminary studies have investigated clinical outcomes after transfemoral TAVI without predilation. The aim of this study, therefore, was to compare the periprocedural and 1-year outcomes of patients who underwent TAVI with or without pre-implantation BAV (pre-BAV) using either self-expanding or balloon-expandable prostheses.
Methods
Analyses were based on a retrospective registry of 837 patients who underwent TAVI in a single Italian center from November 2007 to October 2015. We included all patients who had the valve inserted through the transfemoral route and received either the self-expanding CoreValve/Evolut R (Medtronic, Minneapolis, Minnesota) (n = 246) or the balloon-expandable SAPIEN XT/SAPIEN 3 (Edwards Lifesciences, Irvine, California) (n = 271) prostheses. All other valves and access routes were excluded, with a total of 517 patients considered for the analyses. Patients were divided in 2 groups depending on whether predilation was performed. The decision to perform pre-BAV and balloon size for predilation was left to operator’s discretion. Data were prospectively recorded but retrospectively analyzed. All subjects gave informed consent for data collection and analysis.
The decision to perform TAVI was based on severity of symptoms, risk evaluation, and contraindications to surgery. All patients were evaluated by a multidisciplinary heart team comprising cardiologists, interventional cardiologists, cardiothoracic surgeons, and cardiac anesthetists. Patient demographics, symptoms, and co-morbidities were documented, and individual risk was calculated by the logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE). Transthoracic echocardiogram was the initial screening examination to evaluate severity of aortic stenosis. All patients with severe aortic stenosis considered for an intervention underwent computed tomography (CT) to evaluate annular size, coronary arteries, and peripheral access sites. Coronary angiography was selectively performed in patients with abnormal coronaries or suboptimal CT visualization. Native aortic valve calcification was classified and graded using a semi-quantitative scoring system on a scale of 1 to 4 based on severity as previously described. The TAVI procedure was performed either under conscious sedation (n = 490) or general anesthesia (n = 27). During the procedure, all patients received intravenous heparin to increase the activated clotting time >250 seconds.
The primary objective of the study was to evaluate 1-year major adverse cardiac and cerebrovascular events (MACCE) and mortality in patients who underwent transfemoral TAVI with or without pre-BAV. MACCE were defined as all-cause death, myocardial infarction (MI), and stroke. Secondary objectives included the descriptions of peri-procedural results and 30-day outcome according to the Valve Academic Research Consortium-2 criteria. Paravalvular aortic leakage (PVL) was defined according to the latest recommendations from the European Society of Cardiology. Follow-up was performed by either telephone contact or outpatient clinic visits.
Continuous variables are presented as mean ± SD, and differences in continuous variables between groups were compared with the unpaired Student’s t test or Mann-Whitney U test. Categorical variables are presented as numbers and percentages and tested by the chi-square test or Fisher’s exact test. Cumulative event rates in each group were analyzed using the Kaplan-Meier method, and the rate differences between the groups were estimated using the log-rank test. Multivariate Cox regression was performed to identify independent predictors of MACCE. All the variables with a univariate p <0.10 or if they had clinical relevance (pre-BAV) were subsequently entered into the final model. Results of the Cox regression model are presented as hazard ratio with 95% confidence interval. All reported p values were 2 sided, and a p <0.05 was considered statistically significant. Propensity score matching was used to adjust for differences in baseline characteristics and potential confounders (full methods available online as Supplementary Material ). All statistical analyses were performed using the SPSS software (version 21.0; SPSS Inc., Chicago, Illinois).
Results
Table 1 depicts the baseline characteristics of the study population. A total of 517 patients were included in the analysis (n = 326 pre-BAV, n = 191 without pre-BAV). In the 2 groups, there were no significant differences in multiple baseline variables including age, gender, serum creatinine, logistic EuroSCORE, New York Heart Association (NYHA) class 3 to 4, ejection fraction, and chronic obstructive pulmonary disease ( Table 1 ). Patients without pre-BAV had lower body mass indexes and were more likely to have had a previous percutaneous coronary intervention, whereas those with pre-BAV had higher mean and peak aortic gradients and higher grades of aortic valve calcification. The devices implanted included the Medtronic CoreValve (sizes 23, 26, 29, or 31 mm) (n = 216), Medtronic Evolut R (sizes 23, 26, or 29 mm) (n = 30), Edwards SAPIEN XT (sizes 23, 26, or 29 mm) (n = 210), and Edwards SAPIEN 3 (sizes 23, 26, or 29 mm) (n = 61). Patients who had pre-BAV were more likely to have balloon-expandable valves implanted, whereas if pre-BAV was not performed, a self-expanding device was more commonly used.
| Variable | Predilation (n=326) | No Predilation (n=191) | p value |
|---|---|---|---|
| Age (years) | 79.9 ± 7.4 | 80.8 ± 7.5 | 0.18 |
| Men | 130 (39.9%) | 60 (31.4%) | 0.05 |
| Body mass index (kg/m 2 ) | 26.1 ± 4.7 | 25.2 ± 4.5 | 0.03 |
| Serum creatinine (mg/dL) | 1.4 ± 1.4 | 1.3 ± 0.8 | 0.06 |
| Diabetes mellitus | 93 (28.7%) | 53 (28.5%) | 0.96 |
| Atrial fibrillation | 97 (30.6%) | 65 (34.9%) | 0.31 |
| Cerebrovascular disease | 50 (15.3%) | 22 (11.6%) | 0.24 |
| Coronary artery disease | 125 (38.5%) | 88 (46.8%) | 0.07 |
| Prior coronary artery bypass grafting | 60 (18.4%) | 33 (17.3%) | 0.75 |
| Prior percutaneous coronary intervention | 55 (16.9%) | 48 (25.1%) | 0.02 |
| Peripheral vascular disease | 68 (20.9%) | 42 (22.5%) | 0.67 |
| Chronic obstructive pulmonary disease | 84 (26.1%) | 40 (21.6%) | 0.26 |
| Logistic EuroSCORE (%) | 20.6 ± 15.4 | 21.9 ± 17.1 | 0.41 |
| New York Heart Association class 3-4 | 193 (59.2%) | 117 (61.6%) | 0.60 |
| Echocardiographic findings | |||
| Ejection fraction (%) | 52.5 ± 13.0 | 50.6 ± 13.6 | 0.12 |
| Mean gradient (mmHg) | 54.4 ± 15.8 | 48.7 ± 15.4 | <0.001 |
| Peak gradient (mmHg) | 84.9 ± 22.9 | 77.5 ± 24.0 | <0.01 |
| Aortic valve area (cm 2 ) | 0.7 ± 0.2 | 0.8 ± 0.2 | 0.24 |
| Aortic annulus diameter (mm) | 23.1 ± 2.0 | 22.9 ± 3.9 | 0.60 |
| Aortic valve calcification | |||
| Grade 1-2 | 114 (47.7%) | 90 (60.4%) | 0.02 |
| Grade 3-4 | 125 (52.3%) | 56 (37.6%) | 0.01 |
| Valve type | |||
| Self-expanding valves | 115 (35.3%) | 131 (68.6%) | <0.001 |
| Balloon-expandable valves | 211 (64.7%) | 60 (31.4%) | <0.001 |
In the no-pre-BAV group, the total contrast volume was lower and the postdilation rate was higher (35.6% vs 21.5%, p <0.001). There were no significant differences in the periprocedural complications of death, stroke, transient ischemic attack, MI, coronary obstruction, new-onset atrial fibrillation, emergency surgery, cardiac tamponade, or moderate/severe PVL. Vascular complications occurred at a higher rate in patients who underwent pre-BAV, and this was because of complications requiring percutaneous intervention ( Table 2 ).
| Variable | Predilation (n=326) | No Predilation (n=191) | p value |
|---|---|---|---|
| Fluoroscopy time (minutes) | 27.7 ± 16.0 | 28.7 ± 14.6 | 0.50 |
| Contrast volume (mL) | 155.0 ± 93.0 | 134.1 ± 85.0 | 0.01 |
| Postdilation | 70 (21.5%) | 68 (35.6%) | <0.001 |
| Post-TAVI moderate/severe paravalvular leakage | 50 (15.3%) | 38 (19.9%) | 0.18 |
| Peri-procedural complications | |||
| Death | 2 (0.6%) | 0 | 0.53 |
| Stroke | 1 (0.3%) | 4 (2.1%) | 0.07 |
| Transient ischemic attack | 3 (0.9%) | 0 | 0.30 |
| Myocardial infarction | 2 (0.6%) | 0 | 0.53 |
| Coronary obstruction | 3 (0.9%) | 1 (0.5%) | 1.00 |
| New-onset atrial fibrillation | 19 (5.8%) | 13 (6.8%) | 0.66 |
| Emergency surgery | 3 (0.9%) | 1 (0.5%) | 1.00 |
| Cardiac tamponade | 6 (1.8%) | 3 (1.6%) | 1.00 |
| Vascular complications | 36 (11.0%) | 10 (5.2%) | 0.03 |
| Surgical intervention | 12 (3.7%) | 5 (2.6%) | 0.51 |
| Percutaneous intervention | 24 (7.4%) | 5 (2.6%) | 0.02 |
The 30-day MACCE rate was higher in the no-pre-BAV group ( Table 3 ), and this was driven by a higher incidence of stroke (3.7% vs 0.3%, p <0.01). The stroke rate within 24 hours from TAVI was 2.1% (n = 4) in the no-pre-BAV group and 0.3% (n = 1) in the pre-BAV group (p = 0.07), whereas only 3 more strokes occurred in the no-pre-BAV group in the 1- to 30-day interval. The 30-day bleeding complications and permanent pacemaker implantation were similar between both groups.
| Variable | Predilation (n=326) | No Predilation (n=191) | p value |
|---|---|---|---|
| 30-day major adverse cardiac and cerebrovascular event | 11 (3.4%) | 14 (7.3%) | 0.04 |
| Death | 8 (2.5%) | 9 (4.7%) | 0.17 |
| Myocardial infarction | 2 (0.6%) | 2 (1.0%) | 0.63 |
| Stroke | 1 (0.3%) | 7 (3.7%) | <0.01 |
| 30-day bleeding | 84 (25.8%) | 51 (26.7%) | 0.82 |
| Life-threatening | 9 (2.8%) | 6 (3.1%) | 0.80 |
| Major | 57 (17.5%) | 27 (14.1%) | 0.32 |
| Minor | 28 (8.6%) | 22 (11.5%) | 0.28 |
| 30-day permanent pacemaker implantation | 42 (12.9%) | 26 (13.6%) | 0.81 |
No differences in both MACCE and mortality were present at 12 months between both groups ( Table 4 ). The 1-year stroke rate was significantly higher in the no-pre-BAV group (3.7% vs 1.4%, log-rank test p = 0.03), but all the 7 strokes in the no-pre-BAV group occurred within the 30-day interval, with no more events occurring from 30 days to 12 months. As shown in Figure 1 , there was a nonsignificant trend toward a favorable outcome in the pre-BAV group in both MACCE and mortality at 1 year.
| Variable | Overall Cohort | Balloon-expandable Valves | Self-expanding Valves | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Predilation (n=326) | No Predilation (n=191) | Overall p value ∗ | Predilation (n=211) | No Predilation (n=60) | Overall p value ∗ | Predilation (n=115) | No Predilation (n=131) | Overall p value ∗ | |
| Major adverse cardiac and cerebrovascular event | 0.15 | 0.35 | 0.10 | ||||||
| 3 months | 15 (4.6%) | 18 (10.5%) | 8 (3.8%) | 1 (2.0%) | 7 (6.1%) | 17 (14.0%) | |||
| 1 year | 39 (12.4%) | 24 (16.4%) | 25 (12.4%) | 2 (8.5%) | 14 (12.4%) | 22 (19.8%) | |||
| All-cause death | 0.20 | 0.58 | 0.21 | ||||||
| 3 months | 12 (3.7%) | 13 (7.9%) | 6 (2.9%) | 1 (2.0%) | 6 (5.2%) | 12 (10.2%) | |||
| 1 year | 35 (11.2%) | 20 (14.7%) | 22 (11.0%) | 2 (8.5%) | 13 (11.6%) | 18 (17.2%) | |||
| Stroke | 0.03 | 0.67 | 0.06 | ||||||
| 3 months | 1 (0.3%) | 7 (3.7%) | 1 (0.5%) | 1 (2.0%) | 0 | 6 (4.6%) | |||
| 1 year | 4 (1.4%) | 7 (3.7%) | 3 (1.5%) | 1 (2.0%) | 1 (1.1%) | 6 (4.6%) | |||
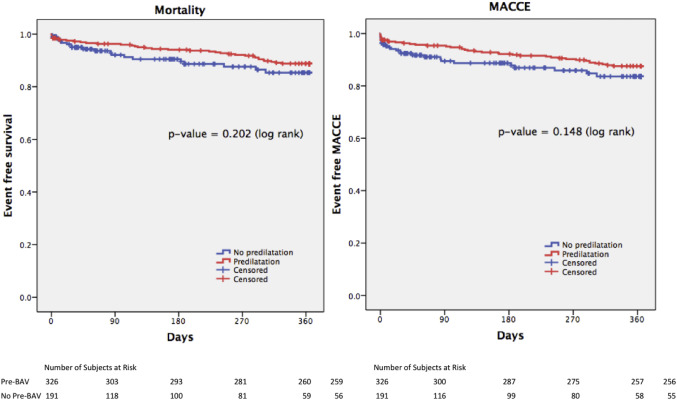
After univariate analysis ( Table 5 ), serum creatinine, logistic EuroSCORE, NYHA class 3 to 4, ejection fraction, and postdilation were predictors of MACCE at 1 year. After adjusting for baseline variables, multivariable analysis revealed serum creatinine, logistic EuroSCORE, NYHA class 3 to 4, and postdilation as independent predictors of MACCE. As postdilation remained a strong predictor of MACCE at 1 year, we divided and analyzed the baseline characteristics of patients on whether postdilation was performed. Apart from pre-BAV and valve type, no other differences were found between both groups ( Supplementary Table 1 ). The postdilation group was associated with a higher 30-day MACCE rate, driven by a higher incidence of death and MI ( Supplementary Table 2 ); in addition, the mortality and MACCE rate findings persisted to 1 year ( Supplementary Figure 1 and Supplementary Table 3 ).
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Hazard Ratio (95% Confidence Interval) | p value | Hazard Ratio (95% Confidence Interval) | p value | |
| Age | 1.02 (0.98-1.06) | 0.29 | ||
| Men | 1.37 (0.83-2.25) | 0.22 | ||
| Body mass index | 0.98 (0.92-1.03) | 0.40 | ||
| Serum creatinine | 1.24 (1.12-1.38) | <0.001 | 1.27 (1.11-1.44) | <0.001 |
| Diabetes mellitus | 1.40 (0.83-2.37) | 0.20 | ||
| Atrial fibrillation | 1.23 (0.72-2.10) | 0.45 | ||
| Cerebrovascular disease | 1.11 (0.57-2.19) | 0.76 | ||
| Coronary artery disease | 1.38 (0.84-2.27) | 0.20 | ||
| Prior coronary artery bypass grafting | 0.92 (0.48-1.75) | 0.79 | ||
| Prior percutaneous coronary intervention | 1.04 (0.57-1.91) | 0.90 | ||
| Peripheral vascular disease | 1.23 (0.70-2.18) | 0.47 | ||
| Chronic obstructive pulmonary disease | 1.19 (0.68-2.08) | 0.55 | ||
| Logistic EuroSCORE | 1.03 (1.02-1.05) | <0.001 | 1.02 (1.01-1.04) | <0.01 |
| New York Heart Association class 3-4 | 2.47 (1.34-4.55) | <0.01 | 2.21 (1.07-4.60) | 0.03 |
| Ejection fraction | 0.98 (0.96-1.00) | 0.02 | 1.00 (0.98-1.02) | 0.95 |
| Mean gradient | 0.99 (0.97-1.01) | 0.21 | ||
| Peak gradient | 1.00 (0.98-1.01) | 0.41 | ||
| Aortic valve area | 1.04 (0.31-3.53) | 0.95 | ||
| Aortic annulus diameter | 1.06 (0.98-1.15) | 0.14 | ||
| Aortic valve calcification grade 3-4 | 1.02 (0.98-1.06) | 0.29 | ||
| Balloon versus self-expanding valve | 0.66 (0.40-1.09) | 0.10 | 1.06 (0.58-1.94) | 0.86 |
| Predilation | 0.69 (0.41-1.15) | 0.15 | 0.90 (0.49-1.68) | 0.86 |
| Postdilation | 2.33 (1.41-3.82) | <0.01 | 2.50 (1.42-4.39) | <0.01 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


