Little is known about the intravascular ultrasound (IVUS) minimal lumen area (MLA) criteria and their accuracy in lesion subsets assorted according to vessel type, lesion location, vessel size, and clinical settings. We therefore assessed the accuracy of subgroup-specific cut-off values in predicting fractional flow reserve (FFR) <0.80. In total 692 consecutive patients with 784 coronary lesions were assessed by IVUS and FFR before intervention. All patients had ≥1 target vessel with a de novo lesion (30% to 90% diameter stenosis). For prediction of FFR <0.80 in the group overall, the best cut-off value of MLA was 2.4 mm 2 (sensitivity 84% and specificity 63%). Overall diagnostic accuracy was only 69%. In the subgroup analysis, the MLA cutoff was 2.4 mm 2 for the left anterior descending coronary artery, 1.6 mm 2 for the left circumflex coronary artery, and 2.4 mm 2 for the right coronary artery. By lesion location, the optimal cutoff was 2.6 mm 2 for proximal, 2.3 mm 2 for mid, and 1.9 mm 2 for distal segments. Furthermore, the cutoffs were 3.2 mm 2 in lesions with a larger RLD >3.5 mm and 1.9 mm 2 in lesions with a smaller RLD <2.75 mm. Nevertheless, diagnostic accuracies of all subgroup-specific criteria were <80%. In conclusion, because IVUS-measured MLA is only 1 of many factors affecting coronary flow hemodynamics, even subgroup-specific criteria were inaccurate in identifying ischemia-inducible stenosis. In conclusion, direct functional assessment is therefore essential in guiding treatment strategies for coronary lesions.
Although percutaneous coronary intervention for ischemia-inducing coronary stenosis leads to favorable clinical outcomes, deferral of revascularization may be preferable for lesions without functional significance. As a standard tool for lesion-specific physiologic assessment, fractional flow reserve (FFR) <0.80 has been validated to identify lesions with inducible ischemia. To integrate anatomic and physiologic measurements, an intravascular ultrasound (IVUS)-defined minimum lumen area (MLA) <4.0 mm 2 has been used to predict the functional significance of stenosis. This cutoff, however, may overestimate the actual functional significance of stenosis and may increase the rate of unnecessary percutaneous coronary intervention. Recently, MLAs of 2.1 to 2.75 mm 2 have been suggested as more realistic for the prediction of abnormal FFR. Little is known, however, about optimal MLA criteria and their accuracies in lesion subsets assorted according to involved vessel, lesion location, vessel size, vascular territories of the myocardium, and specific clinical situations. We therefore evaluated whether subgroup-specific MLA cutoffs could accurately predict FFR <0.80.
Methods
From November 2009 through June 2011, 692 consecutive patients with 784 coronary lesions underwent IVUS and invasive physiologic assessment before intervention and were included in the current analysis. All patients were 35 to 85 years old and had ≥1 target vessel with a de novo lesion (30% to 90% diameter stenosis by visual estimation). Patients were excluded if they had multiple stenoses (diameter stenosis >30% by visual estimation) within a single target vessel, bypass graft lesions, significant left main coronary artery disease, side branch lesions, in-stent restenosis, previous percutaneous coronary intervention at the target vessel, culprit vessels in the setting of a myocardial infarction, Thrombolysis In Myocardial Infarction grade <3 flow, and angiographic thrombi-containing lesions. We also excluded patients with an acute myocardial infarction and scarred myocardium or regional wall motion abnormality of the studied vessel territories. This study was approved by the institutional review board and all patients provided written informed consent.
Qualitative coronary angiographic analysis was performed using standard techniques with automated edge-detection algorithms (CAAS-5, Pie-Medical, Maastricht, The Netherlands) in the angiographic analysis center of the Cardiovascular Research Foundation, Seoul, Korea. According to the SYNTAX classification, coronary segments consisted of the right coronary artery (segments 1, 2, and 3), left anterior descending coronary artery (segments 6, 7, and 8), and left circumflex coronary artery (LCx; segments 11 and 13).
After equalization with a guidewire sensor positioned at the guiding catheter tip, a 0.014-inch pressure guidewire (Radi, St. Jude Medical, Uppsala, Sweden) was advanced distal to the stenosis. FFR was measured at maximal hyperemia induced by an infusion of adenosine 140 μg/kg/min through a central vein. Hyperemic pressure pull-back recordings were performed as previously described. Stenosis was considered functionally significant when FFR was <0.80.
After FFR assessment, IVUS imaging was performed after intracoronary administration of nitroglycerin 0.2 mg using a motorized transducer pullback (0.5 mm/s) and a commercial scanner (Boston Scientific/SCIMED, Minneapolis, Minnesota) consisting of a rotating 40-MHz transducer within a 3.2Fr imaging sheath. Using computerized planimetry (EchoPlaque 3.0, Indec Systems, Mountain View, California), off-line quantitative IVUS analysis was performed as previously described in a core laboratory at the Asan Medical Center. Proximal and distal reference segments were selected within 5 mm proximal and distal to the lesion. Average proximal and distal reference external elastic membrane and reference lumen areas and average reference lumen diameter (RLD) were determined. MLA and external elastic membrane area were measured at the site of the smallest lumen. Plaque burden at the MLA site was calculated as (external elastic membrane area minus lumen area)/external elastic membrane area × 100 (percentage). Percent area stenosis was calculated as (reference lumen area minus MLA)/reference lumen area × 100 (percentage).
All statistical analyses were performed using SAS 9.1 (SAS Institute, Cary, North Carolina) or SPSS 10.0 (SPSS, Inc., Chicago, Illinois). Data were analyzed on a per-patient and per-lesion basis for the corresponding calculations. All values are expressed as mean ± 1 SD (continuous variables) or count and percentage (categorical variables). For per-patient data, continuous variables were compared using unpaired t test or nonparametric Mann–Whitney test; categorical variables were compared using chi-square statistics or Fisher’s exact test. For per-lesion data, a logistic generalized estimated equation model with robust SEs that accounted for clustering between lesions in the same subject were created. To determine independent predictors of FFR <0.8 as a continuous and binary variable, we used a linear mixed model and a multivariable logistic generalized estimated equation model with robust SEs, respectively.
Receiver operating curves were analyzed to assess the best cut-off values of IVUS parameters predicting FFR <0.80 with maximal accuracy using MedCalc (MedCalc, Mariakerke, Belgium). The optimal cutoff was calculated using the Youden index. For each cutoff value, we determined the sensitivity, specificity, positive predictive value, and negative predictive value with 95% confidence intervals. Multivariable logistic regression analysis was also performed to determine independent predictors of FFR <0.8. A p value <0.05 was considered statistically significant.
Results
Table 1 presents a summary of baseline clinical characteristics in 692 patients with 784 lesions. FFR at baseline and maximal hyperemia were 0.95 ± 0.05 and 0.83 ± 0.09, respectively. FFR <0.80 at maximum hyperemia was observed in 227 (29%) lesions.
| Age (years) | 62 ± 10 |
| Men | 498 (72%) |
| Ejection fraction (%) | 61 ± 6 |
| Diabetes mellitus | 224 (32%) |
| Hypertension | 409 (59%) |
| Smoker | 339 (49%) |
| Hyperlipidemia ⁎ | 452 (65%) |
| Previous percutaneous coronary intervention | 77 (11%) |
| Clinical manifestation | |
| Stable angina pectoris | 540 (78%) |
| Unstable angina pectoris | 152 (22%) |
⁎ Defined as total cholesterol >200 mg/dl or receiving antilipidemic treatment.
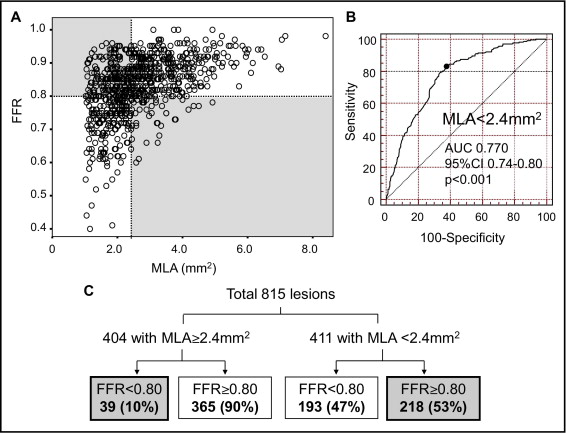
Quantitative angiographic and IVUS findings were presented in Table 2 . Overall, the studied vessels were the left anterior descending coronary artery in 528 (67%), LCx in 68 (9%), and right coronary artery in 188 (24%). Lesion location was proximal in 285 (36%), mid in 405 (52%), and distal in 94 (12%) lesions. IVUS-defined plaque rupture was observed in 51 (7%) lesions. The relation between angiographic diameter stenosis and IVUS MLA was significant but largely scattered (r = −0.445, p <0.001).
| Total | FFR <0.80 | FFR ≥0.80 | |
|---|---|---|---|
| Angiographic data | |||
| SYNTAX no 6 (proximal left anterior descending coronary artery) | 180 (23%) | 66 (29%) | 114 (21%) |
| SYNTAX 7 (mid left anterior descending coronary artery) | 323 (41%) | 108 (48%) | 215 (39%) |
| SYNTAX 8 (distal left anterior descending coronary artery) | 25 (3%) | 10 (5%) | 15 (3%) |
| SYNTAX 11 (proximal left circumflex coronary artery) | 27 (4%) | 3 (1%) | 24 (4%) |
| SYNTAX 13 (distal left circumflex coronary artery) | 41 (5%) | 6 (3%) | 35 (6%) |
| SYNTAX 1 (proximal right coronary artery) | 78 (10%) | 12 (5%) | 66 (12%) |
| SYNTAX 2 (mid right coronary artery) | 82 (10%) | 17 (7%) | 65 (11%) |
| SYNTAX 3 (distal right coronary artery) | 28 (4%) | 5 (2%) | 23 (4%) |
| Proximal reference lumen diameter (mm) | 3.4 ± 0.5 | 3.4 ± 0.5 | 3.4 ± 0.5 |
| Distal reference lumen diameter (mm) | 2.8 ± 0.5 | 2.7 ± 0.5 | 2.9 ± 0.5 ⁎ |
| Averaged reference lumen diameter (mm) | 3.1 ± 0.5 | 3.0 ± 0.5 | 3.1 ± 0.5 ⁎ |
| Minimal lumen diameter (mm) | 1.5 ± 0.4 | 1.3 ± 0.4 | 1.6 ± 0.4 ⁎ |
| Diameter stenosis (%) | 51.0 ± 11.8 | 57.5 ± 11.4 | 48.3 ± 10.8 ⁎ |
| Lesion length (mm) | 19.3 ± 12.0 | 23.4 ± 13.9 | 17.7 ± 10.6 ⁎ |
| Intravascular ultrasound data | |||
| Proximal reference lumen area (mm 2 ) | 8.9 ± 3.2 | 8.1 ± 2.8 | 9.2 ± 3.3 ⁎ |
| Proximal reference vessel area (mm 2 ) | 15.2 ± 4.7 | 15.0 ± 4.5 | 15.3 ± 4.7 |
| Distal reference lumen area (mm 2 ) | 7.3 ± 2.9 | 6.5 ± 2.8 | 7.6 ± 2.9 ⁎ |
| Distal reference vessel area (mm 2 ) | 11.4 ± 4.9 | 10.4 ± 4.8 | 11.7 ± 4.9 ⁎ |
| At minimal lumen area site | |||
| Minimal lumen area (mm 2 ) | 2.7 ± 1.1 | 2.0 ± 0.7 | 2.9 ± 1.2 ⁎ |
| Vessel area (mm 2 ) | 11.0 ± 4.4 | 10.7 ± 4.3 | 11.1 ± 4.4 |
| Mean vessel diameter (mm) | 3.7 ± 0.8 | 3.7 ± 0.8 | 3.7 ± 0.8 |
| Plaque burden (%) | 73.3 ± 12.3 | 79.5 ± 8.8 | 71.0 ± 12.6 ⁎ |
| Area stenosis (%) | 66.1 ± 11.7 | 71.6 ± 9.4 | 64.0 ± 11.8 ⁎ |
⁎ p <0.05 versus lesions with fractional flow reserve <0.80.
FFR was related to age (r = 0.145, p <0.001), angiographic diameter stenosis (r = −0.395, p <0.001), averaged RLD (r = 0.128, p <0.001), lesion length (r = −0.269, p <0.001), IVUS MLA (r = 0.481, p <0.001), plaque burden (r = −0.353, p <0.001), and area stenosis (r = −0.342, p <0.001; Figure 1 ). FFR was significantly decreased in left anterior descending coronary artery lesions versus nonleft anterior descending coronary artery lesions (0.81 ± 0.08 vs 0.86 ± 0.08, p <0.001). FFR <0.80 was observed in 35% of left anterior descending coronary arteries, 13% of LCxs, and 18% of right coronary arteries (p <0.001). Frequency of plaque rupture was higher in lesions with FFR <0.80 versus FFR ≥0.80 (9%, 21/227, vs 5%, 30/557, p = 0.036).
With regard to independent predictors of FFR (as a continuous variable) and FFR <0.8 (as a binary variable), multivariable analyses including age, gender, smoking, hypertension, diabetes, body mass index, acute coronary syndrome, left anterior descending coronary artery location, proximal segment, lesion length, averaged RLD, plaque rupture, MLA, and plaque burden are presented in Table 3 .
| Beta | SE | Adjusted OR | 95% CI | p Value | |
|---|---|---|---|---|---|
| Predictors for fractional flow reserve as continuous variable ⁎ | |||||
| Age | 0.0078 | 0.002 | 0.004–0.011 | <0.001 | |
| Acute coronary syndrome | 0.1045 | 0.040 | 0.027–0.182 | 0.008 | |
| Left anterior descending coronary artery location | −0.3865 | 0.038 | −0.462 to 0.311 | <0.001 | |
| Lesion length | −0.0060 | 0.002 | −0.009 to 0.003 | <0.001 | |
| Plaque rupture | −0.1651 | 0.070 | −0.302 to 0.027 | 0.020 | |
| Minimal lumen area | 0.1854 | 0.018 | 0.149 to 0.222 | <0.001 | |
| Plaque burden | −0.0064 | 0.002 | −0.009 to 0.003 | <0.004 | |
| Predictors for fractional flow reserve <0.8 as binary variable † | |||||
| Age | −0.034 | 0.010 | 0.967 | 0.948–0.986 | 0.001 |
| Left anterior descending coronary artery location | 1.205 | 0.214 | 3.337 | 2.195–5.073 | <0.001 |
| Lesion length | 0.024 | 0.007 | 1.024 | 1.009–1.039 | 0.001 |
| Plaque rupture | 0.967 | 0.374 | 2.629 | 1.263–5.470 | 0.010 |
| Minimal lumen area | −0.897 | 0.148 | 0.408 | 0.305–0.545 | <0.001 |
| Plaque burden | 0.044 | 0.012 | 1.045 | 1.021–1.068 | <0.001 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


