Guidelines for atrial fibrillation (AF) recommend clopidogrel plus aspirin as an alternative stroke prevention strategy in patients in whom warfarin is unsuitable. A Markov model was conducted from a Medicare prospective using data from the Atrial Fibrillation Clopidogrel Trial with Irbesartan for Prevention of Vascular Events–A (ACTIVE-A) trial and other published studies. Base-case analysis evaluated patients 65 years old with AF, a CHADS 2 (congestive heart failure, 1 point; hypertension defined as blood pressure consistently >140/90 mm Hg or antihypertension medication, 1 point; age ≥75 years, 1 point; diabetes mellitus, 1 point; previous stroke or transient ishemic attack, 2 points) score of 2, and a lower risk for major bleeding. Patients received clopidogrel 75 mg/day plus aspirin or aspirin alone. Patients were followed for up to 35 years. Outcomes included quality-adjusted life-years (QALYs), costs (in 2011 American dollars), and incremental cost–effectiveness ratios. Quality-adjusted life expectancy and costs were 9.37 QALYs and $88,751 with clopidogrel plus aspirin and 9.01 QALYs and $79,057 with aspirin alone. Incremental cost–effectiveness ratio for clopidogrel plus aspirin was $26,928/QALY. With 1-way sensitivity analysis using a willingness-to-pay threshold of $50,000/QALY, clopidogrel plus aspirin was no longer cost effective when the CHADS 2 score was ≤1, major bleeding risk with aspirin was ≥2.50%/patient-year, the relative risk decrease for ischemic stroke with clopidogrel plus aspirin versus aspirin alone was <25%, and the utility of being healthy with AF on combination therapy decreased to 0.95. Monte Carlo simulation demonstrated that clopidogrel plus aspirin was cost effective in 55% and 73% of 10,000 iterations assuming willingness-to-pay thresholds of $50,000 and $100,000/QALY. In conclusion, clopidogrel plus aspirin appears cost-effective compared to aspirin alone for stroke prevention in patients with AF with a CHADS 2 of ≥2 and a lower risk of bleeding.
In the Atrial Fibrillation Clopidogrel Trial with Irbesartan for Prevention of Vascular Events–A (ACTIVE-A) trial, clopidogrel plus aspirin was compared to aspirin alone in patients with atrial fibrillation (AF) who were considered unsuitable for therapy with warfarin. Clopidogrel plus aspirin was found to decrease the risk of ischemic stroke by 32% but increase the risk of major intracranial hemorrhage (ICH) by 87% and major extracranial hemorrhage by 51% compared to aspirin alone. Based on these trial results the American College of Cardiology Foundation, American Heart Association, and Heart Rhythm Society added a new (class IIb, level of evidence B) recommendation to their AF treatment guidelines in 2011. The recommendation suggested that in patients with AF in whom oral anticoagulation with warfarin is considered unsuitable because of a patient’s preference or physician’s assessment of the patient’s ability to safely sustain anticoagulation, clopidogrel plus aspirin be considered to decrease the risk of ischemic stroke. In this analysis we sought to estimate the quality-adjusted life years (QALYs), costs, and cost–effectiveness of adding clopidogrel to aspirin in patients with AF in whom warfarin is considered unsuitable.
Methods
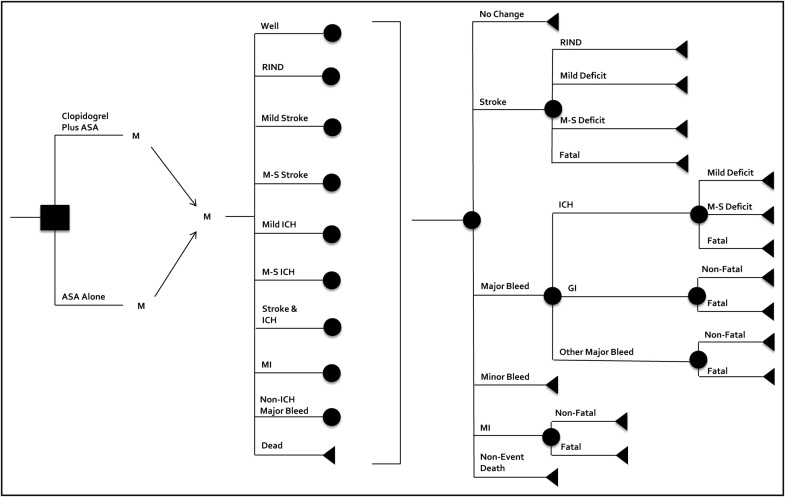
We constructed a Markov cohort transition state model to evaluate the cost–effectiveness of clopidogrel plus aspirin compared to aspirin alone for stroke prevention in patients with AF. The model simulated the progression of a hypothetical cohort of 65-year-old patients with uncomplicated AF, a CHADS 2 (congestive heart failure, 1 point; hypertension defined as blood pressure consistently >140/90 mm Hg or antihypertension medication, 1 point; age ≥75 years, 1 point; diabetes mellitus, 1 point; previous stroke or transient ishemic attack, 2 points) score of 2, and a low risk of bleeding and who initiated pharmacologic stroke prevention with clopidogrel plus aspirin (recommended dose of aspirin 75 to 100 mg/day) or aspirin alone ( Figure 1 ) . Patients were allowed to transition between permanent and temporary health states based on defined transition probabilities that were derived predominantly from the ACTIVE-A trial ( Table 1 ). Other sources of probabilities included published studies of anticoagulation identified through searches of MEDLINE and the Tufts Cost–Effectiveness Analysis Registry and a review of previous economic models. A cycle length of 1 month was used. The model was used to produce estimates of QALYs and costs over a time horizon of 35 years or death (whichever occurred first). The model was run separately for the clopidogrel plus aspirin and aspirin only arms and used to calculate incremental cost–effectiveness ratios (ICERs). The analysis was conducted from the Medicare prospective. Future costs and QALYs were discounted at a rate of 3% annually. The model was built and analyzed using TreeAge Pro 2007 (TreeAge Software, Inc., Williamstown, Massachusetts).
| Variable | Base Case | Range | Reference |
|---|---|---|---|
| Cost (US$) | |||
| Aspirin, monthly | 0.60 | 0–2 | 20 |
| Clopidogrel plus aspirin, monthly | 171 ⁎ | 55–220 | 20 |
| Event cost of minor stroke | 9,619 | 4,500–18,000 | 11,13,21–24 |
| Monthly cost of minor stroke | 2,578 | 1,000–4,500 | 11,13,21–24 |
| Event cost of moderate to severe stroke | 14,283 ⁎ | 11,000–27,500 | 11,13,21–24 |
| Monthly cost of moderate to severe stroke | 5,616 ⁎ | 2,200–9,500 | 11,13,21–24 |
| Event cost of reversible ischemic neurologic event | 6,340 ⁎ | 3,500–13,000 | 11,13,21–24 |
| Event cost of intracranial bleed | 40,237 ⁎ | 16,500–71,000 | 11,13,21–24 |
| Monthly cost of intracranial bleed | 5,935 ⁎ | 2,000–10,500 | 11,13,21–24 |
| Monthly cost of stroke and intracranial bleed | 7,525 ⁎ | 3,500–15,000 | 11,13,21–24 |
| Event cost of gastrointestinal bleed | 5,633 ⁎ | 2,000–9,000 | 13 |
| Event cost of other major bleed | 5,633 ⁎ | 2,000–9,000 | 13 |
| Event cost of minor bleed | 41 ⁎ | 0–200 | 13 |
| Event cost of myocardial infarction | 19,669 | 16,500–24,000 | 25,26 |
| Monthly cost of myocardial infarction | 318 ⁎ | 150–650 | 25,26 |
| Event cost of nonevent death | 6,000 | 3,500–7,500 | 11,13 |
| Annual discount rate (%) | 3 | 0–5 | 11,13 |
| Utility | |||
| Healthy on aspirin | 0.996 | 0.95–1 | 4,10–13 |
| Healthy on clopidogrel plus aspirin | 0.987 | 0.95–1 | estimate |
| Major neurologic event | 0.39 | 0–1 | 4,10–13 |
| Minor neurologic event | 0.75 | 0–1 | 4,10–13 |
| Disutility of major bleed (2 weeks) | 0.16 | 0.10–0.50 | 4,10–13 |
| Disutility of minor bleed (2 days) | 0.16 | 0.10–0.50 | 4,10–13 |
| Myocardial infarction | 0.82 | 0.50–1 | 10 |
| Probabilities | |||
| Baseline rate of stroke on aspirin (%/year) (CHADS 2 score) | 4.5 2 | 0.8–10.9 (0–4) | 3 |
| Relative risk of stroke per decade of life | 1.4 | — | 1 |
| Relative risk of stroke for clopidogrel plus aspirin | 0.68 | 0.57–0.80 | 1 |
| Proportion of strokes that are fatal (%) | 23.2 | 20.2–26.4 | 1 |
| Proportion of strokes that are major (%) | 21.9 | 18.0–25.0 | 1 |
| Proportion of strokes that are minor (%) | 21.0 | 18.0–25.0 | 1 |
| Proportion of strokes with no residual deficit (%) | 33.9 | — | 1 |
| Baseline rate of major hemorrhage on aspirin, per year (%) | 1.30 | 1.02–8.40 | 1 |
| Relative risk of major hemorrhage per decade of life | 1.97 | — | 14 |
| Relative risk of major hemorrhage on clopidogrel plus aspirin | 1.57 | 1.29–1.92 | 1 |
| Percentage of major hemorrhages that are intracranial (%) | 20.1 | 16.5–24.2 | 1 |
| Proportion of intracranial bleeds that are fatal (%) | 36.4 | 28.3–45.2 | 1,14 |
| Proportion of intracranial bleeds that are major (%) | 14.1 | 9.0–21.4 | 1,14 |
| Proportion of intracranial bleeds that are minor (%) | 49.5 | — | 1,14 |
| Proportion of major hemorrhages that are gastrointestinal bleeds (%) | 48.4 | 43.6–53.2 | 1,12,15 |
| Proportion of gastrointestinal bleeds that are fatal (%) | 7.2 | 0–10.0 | 12,13,15 |
| Proportion of major hemorrhages that are other major bleeds (%) | 31.5 | — | 12,13,15 |
| Proportion of other major bleeds that are fatal (%) | 2.1 | 0–4.0 | 12 |
| Rate of minor bleeding on aspirin, per year (%) | 1.4 | 1.1–1.7 | 1 |
| Rate of minor bleeding on clopidogrel plus aspirin, per year (%) | 3.5 | 2.7–4.3 | 1 |
| Baseline rate of myocardial infarction on aspirin, per year (%) | 0.90 | 0.72–1.01 | 1 |
| Relative risk of myocardial infarction per decade of life | 1.3 | — | 13,16 |
| Relative risk of myocardial infarction on clopidogrel plus aspirin | 0.78 | 0.59–1.03 | 1 |
| Proportion of myocardial infarctions that are fatal (%) | 16.6 | 15.8–17.4 | 18 |
| Relative risk of nonevent death with atrial fibrillation | 1.3 | 1.0–1.6 | 7,19 |
| Relative risk of nonevent death with atrial fibrillation and stroke | 2.3 | 1.3–3.0 | 8,19 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


