We sought to assess possible interactions between eplerenone use and a plasma marker of collagen turnover on prognosis in patients after acute myocardial infarction (AMI) and preserved left ventricular (LV) ejection fraction (≥40%). Three hundred three patients with AMI (58 ± 11 years old, 249 men) and preserved systolic LV function were studied prospectively for 24 months. Plasma levels of matrix metalloproteinase-9 (MMP-9) were assessed on day 7 after AMI. Patients were categorized according to whether (n = 201) or not (n = 102) they received eplerenone (25 mg/day) and their baseline MMP-9 levels using the cut-off level suggested by receiver operating characteristics analysis (12.7 ng/ml). Death from cardiovascular causes, nonfatal reinfarction, hospitalization for unstable angina, and development of heart failure symptoms were considered study end points. Eplerenone use was not associated with better prognosis in the entire study group (p = 0.132). However, a significant beneficial eplerenone effect on outcome was observed in patients with low baseline levels of MMP-9 (event-free survival for eplerenone vs noneplerenone arm 65% vs 35%, p = 0.005). Eplerenone had no effect (p = 0.741) in the subgroup of patients with high baseline MMP-9 levels. In conclusion, in patients after AMI with preserved LV systolic function, low baseline levels of MMP-9 identify a subgroup of patients in whom eplerenone use is associated with a survival benefit.
The purpose of the present prospective study was to investigate the effect of eplerenone on the outcome of patients with preserved systolic left ventricular (LV) function (ejection fraction ≥40%) after ST-segment elevation acute myocardial infarction (AMI). Furthermore, we sought to assess possible interactions between eplerenone use and a plasma marker of extracellular matrix turnover (matrix metalloproteinase-9 [MMP-9]) on survival benefit in a group of patients after AMI.
Methods
The present study was designed as an open-label, randomized, parallel-group, nonplacebo-controlled, clinical study. Consecutive patients with ST-segment elevation AMI were recruited if they fulfilled the following inclusion criteria: (1) AMI 1 day to 7 days before enrollment and no aldosterone antagonist treatment and (2) LV ejection fraction ≥40% by transthoracic echocardiography on day 7 after the index event. Exclusion criteria were use of potassium-sparing diuretics, serum creatinine concentration >2.5 mg/dl, and serum potassium concentration >5.0 mmol/L before randomization.
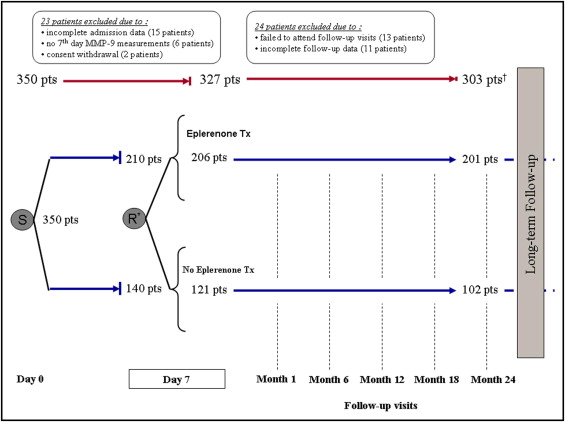
In total 350 consecutive patients, referred to the cardiology department of our university hospital (tertiary referral center) from a single community hospital, were recruited from June 2008 to June 2010. Patients underwent primary thrombolysis for AMI at the community hospital and were subsequently referred for cardiac catheterization and rescue, facilitated, or elective percutaneous coronary intervention (PCI) as indicated. On day 7 after the index event, patients were randomized to receive or not receive eplerenone using a consecutive block randomization schedule (block size 5, allocation ratio 3:2). Randomized patients were then followed for up to 24 months using a standardized protocol that included outpatient visits and telephone contacts. Follow-up contacts included the recording of information about adverse events and concurrent medications and focused on eplerenone treatment adherence and potassium level measurements ( Figure 1 ). The protocol was approved by the institutional ethics committee (96/20-6-2008) and all subjects gave written informed consent.
AMI was diagnosed based on clinical presentation, cardiac enzyme measurements, and electrocardiographic findings according to published guidelines. Patients were included in the study only if they presented within 24 hours from symptom onset.
Eplerenone treatment 25 mg 1 time/day was used. Patients were instructed to interrupt eplerenone treatment if the serum potassium level was ≥5.5 mmol/L. If eplerenone was temporarily stopped because of hyperkalemia, potassium levels were remeasured within 72 hours and eplerenone was restarted if potassium levels were <5.0 mmol/L.
The primary end point of the study was reached when ≥1 of the following occurred: death from cardiovascular causes, nonfatal reinfarction, hospitalization for unstable angina, or decompensation of heart failure. Decompensation was defined as a change in New York Heart Association classification to class ≥II, orthopnea, increased body weight compared to the patient’s known dry body weight, presence of pulmonary rales, S 3 , edema of the lower limbs on clinical examination, pulmonary venous congestion on chest x-ray, or use of new oral diuretics or additional need for intravenous diuretics.
Monitoring for adverse events was performed from the start of study drug prescription until the end of the follow-up period. The following adverse safety outcomes were defined: symptomatic hypotension (systolic blood pressure <100 mm Hg for any reason) requiring study drug discontinuation, reporting of gynecomastia, and hyperkalemia (serum potassium >5.0 mmol/L or requiring study drug discontinuation). Worsening of renal function (absolute increase of serum creatinine concentration ≥0.5 mg/dl or relative increase ≥25% from baseline) was also considered an adverse event.
Peripheral blood samples for measurement of blood chemistry (potassium and renal function) were obtained from all patients on day 7 after the index event and at regular follow-up visits. Blood sampling for MMP-9 assessment was performed only on day 7 and at 12 months from study admission. In addition, in each patient an echocardiographic study was performed on day 7 and at 12 months using standard techniques, in which LV ejection fraction was assessed (Simpson method).
Blood samples were drawn from a peripheral vein in Vacutainer tubes containing ethylenediaminetetraacetic acid as an anticoagulant. Samples were immediately centrifuged at 4,000 rpm for 10 minutes at ambient temperature, and the extracted plasma was stored in aliquots and frozen at −70°C until use. Plasma concentrations of MMP-9 were measured using a commercially available enzyme-linked immunosorbent assay (IBL International, GmbH, Hamburg, Germany). The manufacturer’s reported minimum detectable concentration of MMP-9 was 0.05 ng/ml, and intra-assay and interassay coefficients of variance were 7.3% and 10.2%, respectively.
Association between baseline MMP-9 levels and clinical benefit from eplerenone was analyzed using MMP-9 values as a categorical variable with a cut-off value suggested by receiver operating characteristics curve analysis as having the greatest sensitivity and specificity for the prespecified study end point. Results are presented as median with interquartile range for continues variables and as percentage for categorical data. Normality was tested using the Kolmogorov–Smirnov test. Comparisons between categorical variables were performed by chi-square test or Fisher’s exact test when required. Differences in continuous variables between 2 groups were assessed using the Mann–Whitney U test. Changes in continuous variables between different time-point measurements were carried out using Wilcoxon paired test.
Time-to-event distributions were summarized with Kaplan–Meier curves and compared by log-rank test. For the combined study end point, subjects were censored after the first occurrence and no subjects contributed >1 end point to the analysis. Multivariate Cox proportional hazard analyses were performed to determine independent predictors of the study end point. Adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) were assessed in multivariate Cox models using as cofounders variables that on univariate analysis were shown to significantly affect the occurrence of the prespecified study end point. Variables retained in the final models were chosen with a backward stepwise selection method. Analyses of the study end point were conducted according to the intention-to-treat principle. A p value <0.05 was considered to indicate statistical significance; all tests were 2-sided. IBM PASW-SPSS Statistics 18.0 (SPSS, Inc., Chicago, Illinois) was used for all calculations.
Results
From the initial 350 study participants, 47 patients were excluded because of lack of complete (baseline or follow-up) data monitoring criteria or withdrawal of consent ( Figure 1 ). The remaining 303 patients (mean age 58 ± 11 years, 249 men) constituted the study group (201 patients on eplerenone, 102 not on eplerenone treatment). During the study, 27 patients in the eplerenone group (13%) discontinued the study medication. The reported drug discontinuation reasons are shown in Figure 1 . In patients who discontinued eplerenone because of hyperkalemia (9 patients), only 2 patients (1%) permanently discontinued the study drug, whereas the rest resumed treatment at a later time point. Worsening of renal function or reporting of a symptomatic hypotension event was not documented in any patient under treatment with eplerenone 25 mg.
Baseline characteristics of the study population are presented in Table 1 . Fourteen percent of patients were not revascularized based on coronary angiographic findings. Only 2% of patients required a repeat revascularization procedure during long-term follow-up. Median duration of follow-up was 30 months (interquartile range 24 to 35). Incidence of the combined study end point and its components is presented in Table 2 . Patients who reached the combined end point had a lower ejection fraction (p = 0.001) and were more obese as suggested by their body mass index (p <0.001) compared to patients who remained free of events at follow-up. Furthermore, in patients with ≥1 prespecified end point, revascularization with PCI (p <0.001) and aspirin use (p <0.001) were less common, whereas atrial fibrillation (p <0.001) and use of diuretics (p <0.001) were more common.
| Variable | |
|---|---|
| Age (years) | 58 (50–68) |
| Body mass index (kg/m 2 ) | 29 (26–32) |
| Men/women | 249/54 |
| Risk factors | |
| Hypertension ⁎ | 174 (58%) |
| Diabetes mellitus | 60 (20%) |
| Dyslipidemia † | 138 (46%) |
| Current smoker | 201 (66%) |
| Family history of coronary artery disease ‡ | 150 (50%) |
| Previous myocardial infarction | 30 (10%) |
| Previous stroke | 3 (1%) |
| Atrial fibrillation | 9 (3%) |
| Previous percutaneous coronary intervention | 21 (7%) |
| Previous coronary artery bypass grafting | 6 (2%) |
| Myocardial infarction | |
| Pain-to-door time (minutes) | 150 (75–240) |
| Location of infarct | |
| Anterior/anterolateral | 78 (26%) |
| Inferior/inferolateral | 198 (65%) |
| Lateral | 27 (9%) |
| Right ventricular infarct | 39 (13%) |
| Thrombolysis | 222 (73%) |
| Creatine phosphokinase-MB type (IU/L) | 102 (53–171) |
| Heart rate on admission (beats/min) | 75 (60–85) |
| Systolic blood pressure on admission (mm Hg) | 150 (130–160) |
| Baseline creatinine (mg/dl) | 1 (0.9–1.1) |
| Baseline hemoglobin (g/dl) | 14.8 (13.7–15.6) |
| Baseline estimated glomerular filtration rate (ml/min/1.73 cm 2 ) § | 100 (77–122) |
| Total cholesterol (mg/dl) | 201 (180–220) |
| Low-density lipoprotein cholesterol (mg/dl) | 127 (115–155) |
| High-density lipoprotein cholesterol (mg/dl) | 39 (33–44) |
| Triglycerides (mg/dl) | 129 (92–165) |
| N-terminal propeptide of brain natriuretic peptide at 7 days (pg/ml) | 322 (95–679) |
| Matrix metalloproteinase-9 at 7 days (ng/ml) | 20 (10.5–43) |
| Serum potassium at 7 days (mmol/L) | 4.3 (4.1–4.6) |
| Percutaneous coronary intervention (after admission) | 228 (75%) |
| Coronary artery bypass grafting (after admission) | 33 (11%) |
| Ejection fraction at 7 days by echocardiography (%) | 57 (53–60) |
| Follow-up medication use | |
| Angiotensin-converting enzyme inhibitors | 69 (23%) |
| Angiotensin receptor blockers | 180 (59%) |
| β Blockers | 255 (84%) |
| Calcium channel blockers | 30 (10%) |
| Nitrates | 24 (8%) |
| Diuretics | 27 (9%) |
| Statins | 288 (95%) |
| Fibrates | 10 (3%) |
| Ezetimibe | 26 (9%) |
| ω-3 fatty acids | 27 (9%) |
| Aspirin | 291 (96%) |
| P2Y12 inhibitors | 261 (86%) |
| Anticoagulants | 3 (1%) |
| Antidiabetics | 54 (18%) |
| Insulin | 21 (7%) |
⁎ Defined as systolic blood pressure ≥140 mm Hg, or diastolic blood pressure ≥90 mm Hg, or receiving antihypertensive treatment.
† Defined as total cholesterol >200 mg/dl or receiving hypolipidemic treatment.
‡ Defined as evidence of coronary artery disease in first-degree relatives <55 years old in men and <65 years old in women.
| Study End Point | Study Population | Eplerenone | No Eplerenone |
|---|---|---|---|
| (n = 303) | (n = 201) | (n = 102) | |
| Combined end point ⁎ | 93 (31%) | 57 (28%) | 36 (35%) |
| Cardiovascular death | 9 (3%) | 6 (3%) | 3 (3%) |
| Nonfatal myocardial infarction/unstable angina | 18 (6%) | 9 (4%) | 9 (9%) |
| Heart failure decompensation | 81 (27%) | 51 (25%) | 30 (29%) |
⁎ For the combined study end point, subjects contributed only 1 time.
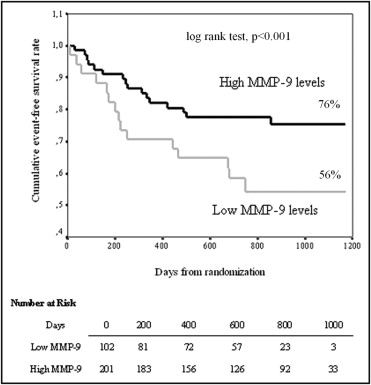
The study population was dichotomized using baseline (day 7 after index event) MMP-9 concentrations with a cut-off value (derived from receiver operating characteristics curve analysis) of 12.7 ng/ml (low MMP-9 levels, n = 102; high MMP-9 levels, n = 201). Of interest, use of eplerenone was similar in the 2 groups (low vs high MMP-9 68% vs 66%, p = 0.797). Low baseline MMP-9 levels were associated with higher mortality and morbidity as suggested by Kaplan–Meier analysis ( Figure 2 ). Cox regression analysis adjusted for all variables that significantly affected the occurrence of the prespecified study end point confirmed that low MMP-9 baseline levels were independently associated with a worse prognosis (HR 2.4, 95% CI 1.6 to 3.6, p <0.001).