Despite the several years of studies, no factor that could reduce the restenosis rate without significant limitations has been introduced. The aim of the present study was to evaluate the influence of low-power 808-nm laser illumination of coronary vessels after percutaneous angioplasty in preventing restenosis. The procedure of laser intravascular illumination was performed on 52 patients (laser group), and another 49 patients formed the control group. All patients were monitored for major adverse cardiac events (MACE) at the 6- and 12-month follow-up points. The MACE rate after 6 and 12 months was 7.7% in the laser group at both points. The MACE rate was 14.3% and 18.5% at 6 and 12 months of follow-up in the control group, respectively (p = NS). Follow-up coronary angiography was performed after 6 months. The difference in the restenosis rate was insignificant (15.0% vs 32.4%); however, significant differences were observed in the minimal lumen diameter (2.18 ± 0.70 vs 1.76 ± 0.74 mm; p <0.05), late lumen loss (0.53 ± 0.68 vs 0.76 ± 0.76 mm; p <0.01), and the late lumen loss index (0.28 ± 0.39 vs 0.46 ± 0.43; p <0.005) in favor of the laser group. In conclusion, the new therapy seemed effective and safe. Marked differences between late loss, late loss index, and minimal lumen diameter were observed. The late lumen loss in the laser group was only slightly greater than that in studies of drug-eluting stents, and MACE rate remained within very comparable ranges. This suggests that intravascular laser illumination could bring advantages comparable to those of drug-eluting stents without the risk of late thrombosis.
Restenosis represents the principal problem associated with the long-term efficacy of percutaneous coronary intervention (PCI). The application of intravascular low-power laser illumination (LPLI) in PCI constitutes one of potential methods to reduce the occurrence of restenosis. The results of in vitro, animal model, and clinical studies have seemed to confirm the hypothesis and have pointed to the safety of such procedures in humans. The present study, conducted using randomized, angiographically controlled, clinical material, evaluated the efficacy of LPLI in preventing restenosis.
Methods
After approval of the appropriate bioethical commission, the clinical study was performed on a group of patients with stable coronary heart disease who had undergone PCI. The lesion to be dilated was 10 to 20 mm long and involved native coronary arteries with a diameter not <2.5 mm. Those with a totally occluded or left main artery and those with ostial, restenotic, thrombotic, or strongly calcified lesions were excluded from the present study. Balloon angioplasty was performed in all patients. If the effect of the procedure was not satisfactory (>30% of residual stenosis), it was followed by bare metal stent implantation. The possibility of drug-eluting stent implantation was not considered. Directly after PCI, a single LPLI of the dilated vessel was performed. Patients with diabetes mellitus or diseases potentially restricting the survival period were excluded from the present study. The included patients were randomized to the laser group or control group using a toss-up randomization process.
A semiconductor laser with a diode pig-tailed using a light fiber (Optel, Opole, Poland) was used for the coronary illumination procedure. The laser was connected to a balloon catheter, with an in-built light fiber, through a subminiature A (SMA 905) fiber connector. The fiber was terminated with a special diffuser. The diffuser, located at the distal end of the catheter with its 20-mm, low-pressure balloon, made it possible to irradiate the coronary artery at the previously performed dilation point with uniform intensity ( Figure 1 ). Radiation with a 808-nm wavelength, 100 mW/cm 2 , and 9 J/cm 2 energy was applied in all cases. The method of intravascular illumination has been precisely described in previous publications.
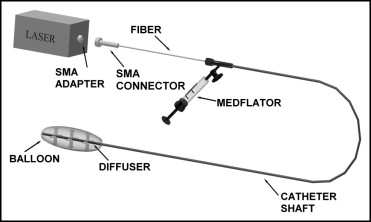
To assess the restenosis rate, control angiography was performed 6 months after PCI. The analyzed variables included restenosis, late loss of vascular lumen (LLL), and the late lumen loss index (LLLI). At the 6- and 12-month follow-up points, all patients were monitored for major adverse cardiac events (MACE)—death, Q- and non–Q-wave myocardial infarction, coronary artery bypass grafting, and target vessel revascularization. Angioplasty and control angiography were conducted using the Innova 2000 angiograph (GE Medical Systems, Fairfield, Connecticut). Quantitative coronary angiographic calculations were performed with the investigator unaware of whether the patient had undergone laser illumination.
All patients received acetylsalicylic acid at a dose of 75 to 150 mg/day and clopidogrel at a dose of 75 mg/day ≥3 days before PCI. After PCI, the patients received acetylsalicylic acid, statins, angiotensin-converting enzyme inhibitors, β blockers, and clopidogrel indefinitely but for ≥2 months. Just before the angioplasty procedure, the patients were given nonfractionated heparin in a single dose of 100 IU/kg body weight. None of the patients received drugs blocking the glycoprotein IIb/IIIa platelet receptors.
Statistical analysis was performed using Statistica, version 6.0 (StatSoft, Krakow, Poland), statistical software. The arithmetic mean (X) and SD were calculated. When quantitative variables manifested normal distribution, the subsequent statistical analysis used Student’s t test or analysis of variance. In the case of a distribution distinct from normal, the independent quantitative variables were analyzed using the Mann-Whitney U test or the nonparametric equivalent of variance analysis, the Kruskal-Wallis analysis of variance test. Quantitative dependent variables were evaluated using Wilcoxon’s test of pair sequences or the nonparametric variance analysis (Friedman’s analysis of variance test). The chi-square test was used for independent qualitative variables and the McNemara test for dependent qualitative variables. Differences at p <0.05 were considered statistically significant.
Results
The study included 101 patients. Of the 101 patients, 29 underwent conventional balloon angioplasty and 72 required additional bare metal stent implantation. Intravascular illumination was performed with LPLI in 52 patients (laser group) and 49 patients formed the control group. The demographic data between the 2 groups were not significantly different ( Table 1 ). Moreover, the 2 groups were not significantly different in the type, spread, or degree of narrowing in the affected lesions, and no difference was seen in the stent implantation frequency or the final effect of the procedure ( Table 2 ).
| Variable | Laser (n = 52) | Control (n = 49) | p Value |
|---|---|---|---|
| Gender | 0.583 | ||
| Women | 12 | 14 | |
| Men | 40 | 35 | |
| Age (years) | |||
| Total | 57 ± 11 | 60 ± 9 | 0.487 |
| Women | 64 ± 12 | 63 ± 9 | 0.746 |
| Men | 55 ± 10 | 57 ± 9 | 0.348 |
| Myocardial infarction | 0.742 | ||
| Total ⁎ | 32 | 29 | |
| In region supplied by dilated artery | 29 | 26 | |
| In another region | 6 | 3 | |
| Canadian Cardiovascular Society class | 0.644 | ||
| I | 12 | 6 | |
| II | 21 | 22 | |
| III | 12 | 14 | |
| IV | 7 | 7 | |
| Arterial hypertension | 24 | 30 | 0.315 |
| Tobacco smoker | 34 | 31 | 0.685 |
⁎ Patients who experienced cardiac infarction in past (in laser group 3 persons experienced infarctions, in control group 1 person experienced 2 infarctions); determination of whether infarction developed in area supplied by dilated artery might have been only tentative in some cases.
| Variable | Laser (n = 52) | Control (n = 49) | p Value |
|---|---|---|---|
| Dilated coronary artery | 0.215 | ||
| Left anterior descending | 29 | 23 | |
| Left circumflex | 11 | 13 | |
| Right | 12 | 13 | |
| Lesions supplied with stents | 0.458 | ||
| Total | 38 | 34 | |
| Left anterior descending | 22 | 20 | |
| Circumflex | 7 | 7 | |
| Right | 9 | 7 | |
| Lesions supplied with balloons | 0.227 | ||
| Total | 14 | 15 | |
| Left anterior descending | 7 | 3 | |
| Circumflex | 4 | 6 | |
| Right | 3 | 6 | |
| Reference diameter of blood vessel (mm) | 3.20 ± 0.44 | 3.11 ± 0.43 | 0.756 |
| Narrowing of blood vessel before percutaneous coronary intervention (%) | 74.1 ± 14.0 | 72.4 ± 12.8 | 0.712 |
| Lesion length (mm) | 13.6 ± 5.0 | 12.0 ± 3.1 | 0.695 |
| Narrowing of blood vessel after percutaneous coronary intervention (%) | 15.3 ± 12.2 | 19.0 ± 11.9 | 0.248 |
| Diameter of applied balloon (mm) | 3.40 ± 0.47 | 3.25 ± 0.38 | 0.746 |
| Pressure used for balloon dilation (atm) | 15.3 ± 3.6 | 14.7 ± 3.5 | 0.648 |
| Length of applied stent (mm) | 19.2 ± 5.5 | 17.6 ± 5.6 | 0.348 |
At the 6-month follow-up point, the total MACE rate was 7.7% in the laser group (3 target vessel revascularizations and 1 non–Q-wave myocardial infarction). The total MACE rate was 14.3% in the control group (5 target vessel revascularizations, 1 death from myocardial infarction, and 1 coronary artery bypass grafting). At the 12-month point, the MACE rate remained unchanged in the laser group (7.7%). However, in the control group, the MACE rate had increased to 18.4% (2 additional coronary artery bypass grafting procedures; p = NS). After 6 months, angiography was performed in the laser group in 40 patients (76.9%). Angiography showed restenosis in 6 patients (15.0%). In the control group, follow-up angiography was performed in 37 patients (75.5%), and 12 (32.4%) had signs of restenosis. The difference between the 2 groups was insignificant. The remaining patients did not report angina complaints and did not agree to undergo follow-up angiography. Thus, 6 (11.5%) of 52 patients in the laser group had developed angiographically confirmed restenosis and 12 (24.5%) of 49 patients in the control group had done so. The intergroup difference was insignificant. The average extent of narrowing did not differ significantly between the 2 groups, either before or directly after PCI. However, the average percentage of narrowing on the follow-up angiogram was significantly greater in the control group. The results obtained during from follow-up angiography were evaluated by dividing the laser and control groups into subgroups of those with and without restenosis. No significant differences were detected between the subgroups without restenosis. In contrast, in the subgroups with restenosis, the narrowing within the lumen was significantly more pronounced in the control group (no previous laser illumination; Table 3 ).
| Variable | Laser (n = 52) | Control (n = 49) | p Value |
|---|---|---|---|
| Mean narrowing on angiogram (%) | 32.0 ± 22.1 | 43.5 ± 23.6 | <0.05 |
| Patients with restenosis (%) | 59.1 ± 22.0 | 71.8 ± 16.3 | <0.01 |
| Patients without restenosis (%) | 27.5 ± 13.0 | 32.2 ± 17.8 | 0.128 |
| Major adverse cardiac events | |||
| At 6 months | 4 (7.7%) | 7 (14.3%) | 0.525 |
| At 12 months | 4 (7.7%) | 9 (18.4%) | 0.266 |
A comparison of the 2 groups did not reveal significant differences in the minimal lumen diameter before or after the procedure nor in the average increase in vascular lumen after PCI. The same was observed when the groups were divided into the subgroups of balloon angioplasty versus stent implantation. In contrast, the minimal lumen diameter at follow-up angiography was significantly greater in the laser group than in the control group. At follow-up angiography, the laser group also had a significantly lower LLL and LLLI. The latter was significantly smaller in both laser subgroups than in the control subgroups. The average values of the vascular measurements are listed in Table 4 .



