The role of the electrophysiologic (EP) study for risk stratification in patients with arrhythmogenic right ventricular cardiomyopathy is controversial. We investigated the role of inducible sustained monomorphic ventricular tachycardia (SMVT) for the prediction of an adverse outcome (AO), defined as the occurrence of cardiac death, heart transplantation, sudden cardiac death, ventricular fibrillation, ventricular tachycardia with hemodynamic compromise or syncope. Of 62 patients who fulfilled the 2010 Arrhythmogenic Right Ventricular Cardiomyopathy Task Force criteria and underwent an EP study, 30 (48%) experienced an adverse outcome during a median follow-up of 9.8 years. SMVT was inducible in 34 patients (55%), 22 (65%) of whom had an adverse outcome. In contrast, in 28 patients without inducible SMVT, 8 (29%) had an adverse outcome. Kaplan-Meier analysis showed an event-free survival benefit for patients without inducible SMVT (log-rank p = 0.008) with a cumulative survival free of an adverse outcome of 72% (95% confidence interval [CI] 56% to 92%) in the group without inducible SMVT compared to 26% (95% CI 14% to 50%) in the other group after 10 years. The inducibility of SMVT during the EP study (hazard ratio [HR] 2.99, 95% CI 1.23 to 7.27), nonadherence (HR 2.74, 95% CI 1.3 to 5.77), and heart failure New York Heart Association functional class II and III (HR 2.25, 95% CI 1.04 to 4.87) were associated with an adverse outcome on univariate Cox regression analysis. The inducibility of SMVT (HR 2.52, 95% CI 1.03 to 6.16, p = 0.043) and nonadherence (HR 2.34, 95% CI 1.1 to 4.99, p = 0.028) remained as significant predictors on multivariate analysis. This long-term observational data suggest that SMVT inducibility during EP study might predict an adverse outcome in patients with arrhythmogenic right ventricular cardiomyopathy, advocating a role for EP study in risk stratification.
The role of the electrophysiologic (EP) study for risk stratification and therapeutic decision making in patients with arrhythmogenic right ventricular cardiomyopathy (ARVC) is controversial. This is partly because of heterogeneous study methods, different EP study protocols, varying sample sizes, and different local thresholds regarding implantable cardioverter defibrillator implantation. The purpose of the present study was to define the predictive value of sustained monomorphic ventricular tachycardia (SMVT) inducibility during an EP study for an adverse outcome in patients with ARVC who were followed up in our tertiary care center.
Methods
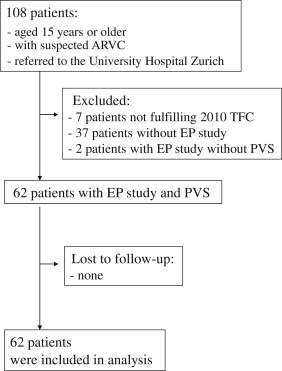
The Zurich ARVC Program (available at www.arvc.ch ) was established in 2011 to provide expert clinical care for patients with ARVC and to study the many facets of this complex disease. Our registry cohort currently includes 101 patients fulfilling the ARVC 2010 Revised Task Force criteria. From this cohort, 62 patients in whom the EP study was performed as a part of routine clinical management and who had a follow-up period of ≥3 months were included in the present study ( Figure 1 ). The local ethical committee approved the present retrospective study. Each participant gave written informed consent before the EP study.
Clinical information regarding demographics, symptoms, 12-lead surface and long-term electrocardiographic findings, and documented arrhythmias were obtained before the EP study. The surface electrocardiograms at the EP study were digitally recorded and independently analyzed by 2 independent arrhythmia specialists who were unaware of the outcome data.
All patients underwent a complete echocardiographic examination at first presentation by an echocardiography specialist. The echocardiographic reports were reviewed for the presence of major or minor Revised Task Force criteria. Right ventricular angiography and/or magnetic resonance imaging were performed at the discretion of the managing physician in charge of the patient. Follow-up for survival data was performed by hospital chart review and telephone interviews of the patients, their relatives, or treating physicians.
The decision to perform an EP study was made on an individual basis, according to the judgment of the treating arrhythmia specialist. The EP study was performed according to a standard protocol, as previously reported. In patients in whom ventricular tachycardia (VT) was not inducible at baseline, isoproterenol was administrated intravenously (≤4 μg/min), followed by application of ≤3 extra stimuli and burst pacing (to a minimum of 250 ms), if deemed clinically indicated by the operators. The EP findings were considered positive if monomorphic VT was sustained or required termination because of hemodynamic compromise. The presence of inducible ventricular fibrillation (VF) or polymorphic VT was not considered a positive EP finding. A right ventricular angiogram was obtained for 41 patients to assess for localized dyskinetic or akinetic outpouchings, as previously described.
Patients were followed up at 6- to 12-month intervals, and data, including invasive and noninvasive investigations, pedigree, device interrogation, and stored electrograms, were obtained throughout the follow-up period.
The role of the EP study in predicting the long-term clinical outcome was compared in 2 patient groups: group 1, patients with an adverse outcome (a composite of cardiac death, heart transplantation, survived sudden cardiac death, VF, sustained VT with hemodynamic compromise and syncope) and group 2, patients with a favorable outcome (all remaining patients), as previously reported. Follow-up was to the date of an adverse outcome or the most recent follow-up evaluation, whichever came first.
Sudden cardiac death and syncope were defined as previously reported. Only syncope with a presumed arrhythmic origin was considered for our analysis. VF was defined as an irregular or regular tachycardia of ventricular origin with a mean cycle length of ≤240 ms. VT was defined as a regular tachycardia of ventricular origin with a mean cycle length of 241 to 600 ms. We differentiated between VT with hemodynamic compromise and VT without hemodynamic compromise, depending on the presence of a symptomatic decrease in the arterial blood pressure and/or (pre)syncope. Nonsustained VT and electrical storm were defined as previously reported. Stored intracardiac electrograms were analyzed to classify arrhythmias. For the evaluation of adherence, the patient was considered nonadherent if a corresponding remark was present in the medical record indicating that the patient was taking antiarrhythmic drugs irregularly or did not follow recommendations regarding restriction from strenuous exercise. Continuous variables are presented as the mean ± SD or median and range or interquartile range, as stated and were compared across groups using a 2-sided unpaired t test or Mann-Whitney U test, as appropriate. Categorical variables are reported as frequencies and percentages and compared between groups using Fisher’s exact test. Cumulative probabilities of survival free of the adverse outcome were determined using the Kaplan-Meier method, and differences in survival between groups were determined using the log-rank test. Uni- and multivariate Cox regression analyses were used to identify independent predictors for an adverse outcome. To account for the differences in arrhythmic risk between patients with and without a previous episode of sudden cardiac death, VF, or sustained VT, Kaplan-Meier plots and Cox regression analyses were stratified for this variable. A p value of <0.05 was considered significant. Statistical analysis was performed using R programming language (R Development Core Team, 2009, Vienna, Austria) and GraphPad Prism, version 5 (GraphPad Software, La Jolla, California).
Results
An EP study with programmed ventricular stimulation was performed in 62 patients at baseline. The patient demographic data and baseline characteristics are summarized in Tables 1 and 2 . All patients were probands. ARVC was classified as “definite” in 44 patients (71%) and “borderline” in 18 patients (29%) according to the 2010 Revised Task Force criteria. The borderline cases fulfilled 1 major and 1 minor criterion (n = 11) or 3 minor criteria (n = 7). In 24 patients (39%), spontaneous sustained VT with hemodynamic compromise or VF was documented and in 20 patients (32%), spontaneous sustained VT without hemodynamic compromise was documented prior to the EP study. Monomorphic VT/VF documented prior to the EP study had a mean cycle length of 287 ± 51 ms (range 181 to 420). An EP study for the evaluation of syncope without documented spontaneous sustained VT or VF was performed in 15 patients (24%). Coronary artery disease was rare (n = 3) and only prevalent in the group with a favorable outcome (p = 0.24 vs an adverse outcome). None of these patients had signs of left ventricular involvement. An implantable cardioverter defibrillator was implanted in 1 of these patients for primary prevention.
| Characteristic | All Patients (n = 62) | Outcome | p Value | |
|---|---|---|---|---|
| Favorable (n = 32) | Adverse (n = 30) | |||
| Age at electrophysiologic study (yrs) | 42.3 ± 13.5 | 44.9 ± 11.3 | 39.2 ± 14.7 | 0.076 |
| Male gender | 42 (68%) | 22 (69%) | 20 (67%) | 1.000 |
| Systolic blood pressure (mm Hg) | 120 (106–128) | 120 (108–130) | 115 (105–126) | 0.178 |
| Diastolic blood pressure (mm Hg) | 75 (70–80) | 79 (70–82) | 75 (68–80) | 0.312 |
| Body mass index (kg/m 2 ) | 24.8 (22.3–26.9) | 25.2 (22.8–26.4) | 24.6 (22–28) | 0.704 |
| Body surface area (m 2 ) | 1.92 ± 0.18 | 1.93 ± 0.17 | 1.91 ± 0.21 | 0.76 |
| Characteristic | All Patients (n = 62) | Outcome | p Value | |
|---|---|---|---|---|
| Favorable (n = 32) | Adverse (n = 30) | |||
| First arrhythmogenic right ventricular cardiomyopathy-related symptom | ||||
| Sudden cardiac arrest | 7 (11%) | 3 (9%) | 4 (13%) | 0.703 |
| Palpitations | 30 (48%) | 14 (44%) | 16 (53%) | 0.612 |
| Presyncope | 18 (29%) | 8 (25%) | 10 (33%) | 0.579 |
| Chest pain | 16 (26%) | 7 (22%) | 9 (30%) | 0.566 |
| Dyspnea | 9 (15%) | 5 (16%) | 4 (13%) | 1.00 |
| Peripheral edema | 1 (2%) | 1 (3%) | 0 | 1.00 |
| Syncope before electrophysiologic study | 31 (50%) | 12 (38%) | 19 (63%) | 0.074 |
| New York Heart Association class at electrophysiologic study | ||||
| I | 43 (70%) | 25 (78%) | 18 (60%) | 0.169 |
| II | 11 (16%) | 4 (13%) | 7 (23%) | 0.329 |
| III | 9 (15%) | 3 (9%) | 6 (20%) | 0.294 |
| IV | 0 | 0 | 0 | 1.00 |
| Sustained ventricular tachycardia with hemodynamic compromise/ventricular fibrillation before electrophysiologic study | 24 (39%) | 8 (25%) | 16 (53%) | 0.036 |
| Sustained ventricular tachycardia without hemodynamic compromise before electrophysiologic study | 20 (32%) | 12 (38%) | 8 (27%) | 0.432 |
| Nonsustained ventricular tachycardia only before electrophysiologic study | 14 (23%) | 5 (16%) | 9 (30%) | 0.23 |
| Left ventricular involvement, ejection fraction <50% | 15 (24%) | 6 (19%) | 9 (30%) | 0.379 |
| Severe right ventricular dysfunction, fractional area change <33% | 30 (48%) | 13 (41%) | 17 (57%) | 0.39 |
| “Definite” arrhythmogenic right ventricular cardiomyopathy per 2010 Task Force criteria | 44 (71%) | 22 (69%) | 22 (73%) | 0.783 |
The baseline EP data are listed in Table 3 . Overall, ventricular tachyarrhythmias were induced in 46 patients (74%). SMVT was induced in 34 patients (55%), and VF in 15 patients (24%; Table 4 ). No sustained polymorphic VT was induced. No difference was seen in patient age at the EP study between those with inducible SMVT and those without (age 41.9 ± 14.2 vs 42.8 ± 12.9 years, p = 0.79). Isoproterenol was used in 7 patients (11%). In this subgroup, SMVT was induced in 3 patients and nonsustained monomorphic VT in 1 patient. The EP study results with isoproterenol were not different from those without (p = 0.38). Monomorphic VT with ≥2 morphologies was inducible in 21 patients (42% of all patients with inducible VT). Of those, 7 patients (14%) displayed 3 morphologies and 1 patient showed 4 different morphologies. The EP study was continued in all patients despite induction and termination of the first monomorphic VT.
| Characteristic | All Patients (n = 62) | Outcome | p Value | |
|---|---|---|---|---|
| Favorable (n = 32) | Adverse (n = 30) | |||
| Spontaneous rhythm at electrophysiologic study | ||||
| Sinus rhythm | 59 (95%) | 30 (94%) | 29 (97%) | 1.00 |
| RR (ms) | 995 ± 218 | 979 ± 194 | 1,013 ± 243 | 0.553 |
| PR (ms) | 163 (136–200) | 168 (149–210) | 160 (133–195) | 0.28 |
| QRS (ms) | 106 (83–120) | 107 (81–119) | 100 (83–125) | 0.95 |
| QTc (ms) | 418 ± 40 | 422 ± 44 | 412 ± 35 | 0.382 |
| AH (ms) | 111 ± 41 | 114 ± 51 | 108 ± 30 | 0.669 |
| HV (ms) | 45 (32–54) | 46 (38–52) | 41 (34–58) | 0.715 |
| Atrioventricular conduction | ||||
| Normal atrioventricular node physiology | 38 (61%) | 20 (63%) | 18 (60%) | 1.00 |
| Dual atrioventricular node physiology | 10 (16%) | 6 (19%) | 4 (13%) | 0.733 |
| Prolonged HV conduction | 7 (11%) | 2 (6%) | 5 (17%) | 0.249 |
| Inducible supraventricular tachycardia | 13 (21%) | 10 (31%) | 5 (17%) | 0.24 |
| Atrioventricular nodal reentrant tachycardia | 1 (2%) | 1 (3%) | 0 | 1.00 |
| Focal atrial tachycardia | 5 (8%) | 4 (13%) | 1 (3%) | 0.355 |
| Atrial fibrillation | 2 (3%) | 2 (6%) | 1 (3%) | 1.00 |
| Atrial flutter | 4 (7%) | 2 (6%) | 3 (10%) | 0.667 |
| Atrioventricular reentrant tachycardia | 1 (2%) | 1 (3%) | 0 | 1.00 |
| Characteristic | All Patients (n = 62) | Outcome | p Value | |
|---|---|---|---|---|
| Favorable (n = 32) | Adverse (n = 30) | |||
| Inducible sustained ventricular tachyarrhythmia | 40 (65%) | 17 (53%) | 23 (77%) | 0.067 |
| Ventricular fibrillation | 15 (24%) | 8 (25%) | 7 (23%) | 1.00 |
| Sustained monomorphic ventricular tachycardia | 34 (55%) | 12 (38%) | 22 (73%) | 0.006 |
| Nonsustained monomorphic ventricular tachycardia | 16 (26%) | 7 (22%) | 9 (30%) | 0.566 |
| Cycle length of monomorphic ventricular tachycardia (ms) | 293 ± 70 | 304 ± 90 | 285 ± 51 | 0.296 |
| Monomorphic ventricular tachycardia with ≥2 morphologies | 21 (34%) | 9 (28%) | 12 (40%) | 0.423 |
| Left bundle branch block morphology | 40 (80%) | 16 (84%) | 24 (77%) | 0.722 |
| Inferior axis | 20 (40%) | 9 (47%) | 11 (36%) | 0.553 |
| Superior axis | 19 (38%) | 8 (42%) | 11 (36%) | 0.766 |
| Indeterminate axis | 1 (2%) | 0 | 1 (3%) | 1.00 |
| Right bundle branch block morphology | 4 (8%) | 2 (11%) | 2 (7%) | 0.629 |
| Origin of ventricular tachycardia | ||||
| Right ventricular outflow tract | 19 (38%) | 6 (32%) | 13 (42%) | 0.556 |
| Right ventricular apex | 13 (26%) | 5 (26%) | 8 (26%) | 1.00 |
| Right ventricular inferoposterior wall | 7 (14%) | 4 (21%) | 3 (10%) | 0.404 |
| Right ventricular septum | 4 (8%) | 2 (11%) | 2 (7%) | 0.629 |
| Right ventricular free wall | 3 (6%) | 2 (11%) | 1 (3%) | 0.549 |
| Left ventricle | 2 (4%) | 0 | 2 (7%) | 0.519 |
| Sustained polymorphic ventricular tachycardia | 0 | 0 | 0 | — |
| Nonsustained polymorphic ventricular tachycardia | 6 (10%) | 5 (26%) | 1 (3%) | 0.078 |
| Interventions | ||||
| Endocardial ventricular tachycardia ablation | 9 (15%) | 2 (6%) | 7 (23%) | 0.077 |
| Surgical right ventricular myectomy | 1 (2%) | 1 (3%) | 0 | 1.00 |
| Flutter isthmus ablation | 5 (8%) | 2 (6%) | 3 (10%) | 0.667 |
| Slow pathway modification | 1 (2%) | 1 (3%) | 0 | 1.00 |
| Atrioventricular nodal ablation | 1 (2%) | 1 (3%) | 0 | 1.00 |
No specific EP finding was predictive of SMVT inducibility. As detected by echocardiography, right ventricular angiography, or magnetic resonance imaging, 52 (66%) monomorphic VTs corresponded to regional wall motion abnormalities. The mean cycle length of induced monomorphic VT was not different between patients with a favorable versus adverse outcome ( Table 4 ). During the EP study, 32 antitachycardia pacing therapies were attempted to terminate SMVT and were successful in 28 cases (88%). Four antitachycardia pacing attempts were not successful, and three of those degenerated from VT into VF. The mean cycle length of SMVT that could be successfully terminated with overdrive stimulation was longer than the mean cycle length of SMVT with unsuccessful antitachycardia pacing (314 ± 14 ms, 95% confidence interval [CI] 286 to 342 vs 265 ± 14 ms, 95% CI 221 to 309; p = 0.03). Accordingly, SMVT with a cycle length of ≥300 ms (<200 beats/min) inferred a >90% probability of successful antitachycardia pacing during the EP study. Antiarrhythmic drugs, including either a β blocker (n = 35 [57%]), sotalol (n = 13 [21%]), amiodarone (n = 7 [11%]), or dronedarone (n = 1 [2%]) were started in 50 patients after the EP study. A combination therapy of a β blocker with sotalol, amiodarone, or dronedarone was initiated in 5 patients (8%).
In total, 48 patients (77%) received an implantable cardioverter defibrillator, 17 for primary and 31 for secondary prevention. An implantable cardioverter defibrillator was implanted in 29 patients (85%) with inducible SMVT. In 5 patients, an implantable cardioverter defibrillator was not implanted despite positive EP study findings; 3 of these patients continued taking antiarrhythmic drugs and 2 patients for whom an implantable cardioverter defibrillator was recommended rejected implantation. Of 14 patients without an implantable cardioverter defibrillator, 11 did not experience an adverse outcome during a median follow-up of 7.0 years (range 1.6 to 31.9). Both patients who had rejected implantable cardioverter defibrillator implantation had an adverse event (sustained VT with hemodynamic compromise and syncope), and the 3 patients who continued taking antiarrhythmic drugs were free of an adverse event during follow-up. One patient with negative EP study findings succumbed to a cardiac death from heart failure without documentation of sustained ventricular arrhythmias. In the subpopulation of 14 patients without an implantable cardioverter defibrillator, only 1 patient was nonadherent (9%) and none had heart failure New York Heart Association functional class III to IV or a left ventricular ejection fraction of ≤35%.
During a median follow-up of 9.8 years (interquartile range 4.4 to 12.5), 30 patients (48%) experienced an adverse outcome ( Table 5 ). The median follow-up was similar between patients with favorable versus adverse outcomes (9.2 years, interquartile range 2.0 to 12.7, vs 10.2 years, interquartile range 4.6 to 12.5; p = 0.57). In patients who only experienced sustained VT without hemodynamic compromise, the median VT cycle length was longer than that in patients with sustained VT with hemodynamic compromise or VF (319 ms, interquartile range 286 to 400, vs 273 ms, interquartile range 240 to 320; p = 0.023). The median interval from the EP study to an adverse event was 13.3 months (range 0.4 to 124.8). One quarter of patients had their initial event ≥3 years after the EP study ( Figure 2 ). An electrical storm was observed in 11 patients (18%) and arrhythmic syncope in 7 (11%). SMVT was inducible in all (91%) but 1 patient, who experienced an electrical storm during follow-up. Of the 34 patients with inducible SMVT, 22 (65%) had an adverse outcome. In contrast, of 28 patients without inducible SMVT, 8 (29%) had an adverse outcome. Inducibility of SMVT was predictive for an adverse outcome with an odds ratio of 2.87 (95% CI 1.27 to 6.46, p = 0.006), positive predictive value of 65% (95% CI 48% to 79%), negative predictive value of 71% (95% CI 45% to 77%), sensitivity of 73% (95% CI 56% to 86%), and specificity of 63% (95% CI 44% to 79%). Overall, the cumulative survival free of adverse events was 76% (95% CI 61% to 95%) and 72% (95% CI 56% to 92%) in the group without inducible SMVT and 42% (95% CI 28% to 64%) and 26% (95% CI 56% to 92%) in the group with inducible SMVT after 5 and 10 years, respectively. On Kaplan-Meier analysis stratified by the inducibility of SMVT, this benefit in event-free survival was significant (p = 0.008; Figure 2 ). Detailed data for the 2 subgroups with (n = 36) and without (n = 26) an episode of sudden cardiac death, VF, or sustained VT prior to the EP study are illustrated by the Kaplan-Meier plot stratified by the inducibility of SMVT and previous sudden cardiac death, VF, or sustained VT ( Figure 2 ). Evidence was seen for a benefit in event-free survival between the groups with and without inducible SMVT stratified by a previous episode of sudden cardiac death, VF, or sustained VT (p = 0.014). Separate Kaplan-Meier analyses for the 2 subgroups also yielded evidence for a benefit in event-free survival in patients without inducible SMVT (p = 0.055 for the subgroup with sudden cardiac death, VF, or sustained VT before the EP study; and p = 0.12 for the subgroup without sudden cardiac death, VF, or sustained VT prior to the EP study). At the last follow-up visit, 57 patients (92%) were alive. Four patients had died from a cardiac cause ( Table 5 ) and one from metastatic cancer. Heart transplantation for progressive heart failure (n = 1) and/or incessant arrhythmias (n = 2) was performed in 3 patients.



