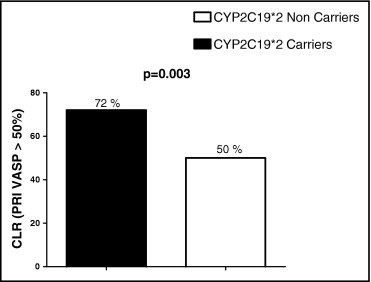
The cytochrome P450 (CYP) 2C19*2 loss-of-function allele has been associated with impaired clopidogrel response and worse prognosis in clopidogrel-treated patients. The benefit of tailored therapy according to platelet function test results remains unclear, and the potential effect of genotypes on this benefit has not been addressed in unstable patients. The present study was designed to evaluate the benefit of tailored therapy with a higher maintenance dose according to CYP2C19 genotypes in patients identified as nonresponders who underwent percutaneous coronary intervention for non–ST-segment elevation acute coronary syndromes. Three hundred forty-six consecutive patients were enrolled and received a loading dose of 600 mg, including 86 *2 carriers (13 homozygotes and 73 heterozygotes) and 260 *2 noncarriers. Clopidogrel response, assessed with platelet reactivity index vasoactive-stimulated phosphoprotein, was significantly affected by genotype, with lower clopidogrel response in CYP2C19*2 allele carriers (p = 0.01). Accordingly, the rate of clopidogrel nonresponse was higher in CYP2C19*2 allele carriers (53% vs 41%, p = 0.04). All clopidogrel nonresponders (n = 151), including 105 *2 noncarriers and 46 *2 carriers, received high 150-mg clopidogrel maintenance doses at discharge to overcome initial low response. After 1 month, high maintenance doses overcame clopidogrel low response in only 44% of the whole population and significantly less frequently in *2 carriers than in noncarriers (28% vs 50%, p = 0.01). In conclusion, higher clopidogrel maintenance doses were able to overcome clopidogrel low response in fewer than half of clopidogrel low responders who underwent percutaneous coronary intervention for non-ST–segment elevation acute coronary syndromes. The benefit of this tailored therapy was significantly reduced in CYP2C19*2 carriers. Therefore, these patients might require alternative strategies with new P2Y 12 blockers.
Dual-antiplatelet therapy with aspirin and clopidogrel is currently the gold standard for patients who undergo percutaneous coronary interventions (PCI) and/or those with non–ST-segment elevation (NSTE) acute coronary syndromes (ACS). Several biologic studies have described a broad interindividual variability of biologic response to clopidogrel. The clinical relevance of such biologic variability has been demonstrated in different clinical settings for ischemic and bleeding complications. Mechanisms underlying this variability of response remain unclear and multifactorial. Clopidogrel is an inactive prodrug that requires several biotransformation steps. After intestinal absorption, clopidogrel biotransformation into its active metabolite is mainly mediated by the hepatic cytochrome P450 (CYP). Recent data have shown that the CYP2C19*2 loss-of-function allele is associated with a marked decrease in platelet response to clopidogrel in unstable patients and an impaired prognosis in clopidogrel-treated patients. The benefit of tailored antiplatelet therapy based on platelet test results in patients identified as nonresponders is still questionable, and randomized studies are ongoing to evaluate its benefits in different settings. Doubling of the clopidogrel maintenance dose may represent an appealing alternative compared to a switch to new P2Y 12 blockers in subjects with poor response profiles. Indeed, higher clopidogrel regimens for loading and maintenance doses have been used to overcome variability of response in biologic studies, and recently, the clinical benefit of such doses has been suggested in patients who undergo PCI for ACS. However, the benefit of tailored therapy with high maintenance doses in unstable patients and the effect of the CYP2C19*2 loss-of-function allele on this benefit have not been properly addressed. Therefore, we designed the present study to assess the benefit of high maintenance doses in low-response patients and the impact of CYP2C19*2 genotypes on this benefit.
Methods
Consecutive patients admitted for NSTE ACS to our institution from July 2008 to January 2010 were eligible for this prospective study if they had undergone successful PCI. NSTE ACS were defined as clinical symptoms compatible with acute myocardial ischemia <12 hours before admission and ≥1 of the following: a new finding of ST-segment changes in ≥2 leads, elevated levels of cardiac markers, or coronary artery disease as documented by a history of revascularization or acute myocardial infarction. The exclusion criteria were a history of bleeding diathesis, persistent ST-segment elevation ACS, New York Heart Association class IV, PCI or coronary artery bypass grafting <3 months, contraindications to antiplatelet therapy, platelet count <100 G/L, and creatinine clearance <25 ml/min. Patients received oral loading doses of aspirin 250 mg and clopidogrel 600 mg ≥12 hours before PCI. Initial platelet parameters were assessed 12 to 24 hours after the loading dose. PCI was performed <24 hours after admission. Anticoagulation was obtained with unfractionated heparin or subcutaneous fondaparinux 2.5 mg/day plus additional unfractionated heparin during PCI. The use of a glycoprotein IIb/IIIa antagonist during PCI was allowed at the operator’s discretion during the procedure.
After initial assessment, patients identified as low responders received higher 150-mg maintenance doses of clopidogrel, and platelet tests were repeated 1 month after hospital discharge at clinical follow-up. Verbal compliance was also systematically checked during this consultation. The study protocol was approved by the ethics committee of our institution, and patients gave written informed consent for participation.
Blood samples for testing clopidogrel response were drawn ≥12 hours after the loading dose of clopidogrel and at 1-month follow-up. Blood was sent immediately to the hemostasis laboratory to determine the vasoactive-stimulated phosphoprotein (VASP) phosphorylation state of whole blood. We used a standardized flow cytometric assay (Platelet VASP; Diagnostica Stago (Biocytex), Asnières, France), which is an adaptation of the method of Schwarz et al previously described. A platelet reactivity index (PRI) VASP (VASP) was calculated from the median fluorescence intensity (MFI) of samples incubated with prostaglandin E 1 (PGE 1 ) or PGE 1 and adenosine diphosphate (ADP) according to the formula PRI VASP = (MFI PGE1 − MFI PGE1+ADP /MFI PGE1 ) × 100. Clopidogrel low response was defined as PRI VASP >50%, as previously proposed.
Genomic deoxyribonucleic acid was extracted from peripheral blood leukocytes by the salting-out method. CYP2C19*2 (rs4244285) genotyping was done using amplification refractory mutation system polymerase chain reaction. The primers (Invitrogen, Carlsbad, California) were as follows: *2 forward: CAg AgC TTg gCA TAT TgT ATC; *2 reverse: TAT CgC AAg CAg TCA CAT AAC; *2 G specific (sens): ACT ATC ATT gAT TAT TTC CCg; and *2 A specific (antisens): gTA ATT TgT TAT ggg TTC CT. The sizes of the polymerase chain reaction products generated are as follows: CYP2C19 *2 forward/reverse: 373 bp, *2 G (normal allele): 283 bp; and *2 A (mutated) allele: 129 bp. Reactions were made in a final volume of 12.5 μl, using 200 nmol/L of primer except for *2 A primer (300 nmol/L), 0.15 U of Taq deoxyribonucleic acid polymerase (Qbiotaq; MP Biomedicals, Solon, Ohio) in its accompanying buffer, 200 μmol/L of accompanying deoxynucleotide triphosphates, and 25 ng of genomic deoxyribonucleic acid. The sequence variation c.681G>A in the CYP2C19 gene was screened to determine the presence of the wild-type allele or 2*. Base numbering and allele definitions followed the nomenclature of the Human Cytochrome P450 Allele Nomenclature Committee ( http://www.cypalleles.ki.se ) according to the recommendations of den Dunnen ( http://www.hgvs.org/mutnomen/ ).
The end points of the present study were (1) the effect of the CYP2C19*2 allele on clopidogrel response after a 600-mg loading dose in patients with NSTE ACS, (2) the benefit of a high 150-mg maintenance dose in clopidogrel low responders after PCI for NSTE ACS, and (3) the effect of CYP2C19*2 genotypes on the benefit of a high maintenance dose in low responders.
Statistical analysis was performed using the GraphPad Prism version 4.00 (GraphPad Software, San Diego, California). Continuous variables were analyzed for a normal distribution using the Shapiro-Wilk test and are expressed as mean ± SD. One-way analysis of variance was used for comparisons across genotypes and to generate p values for trend tests. The Kruskal-Wallis test was used for comparisons across groups and to generate p values for trend tests of variables not normally distributed. Categorical variables are expressed as frequencies and percentages. Comparisons between groups were made using chi-square or Fisher’s exact tests for categorical variables and nonparametric statistical testing or paired Student’s t tests for continuous variables. A p value <0.05 was considered statistically significant.
Results
Among 382 patients screened, 346 unrelated participants were included in our study. Baseline characteristics of the population are included in Table 1 according to clopidogrel response. Genetic distributions of the CYP2C19*2 polymorphisms were as follows: 4% *2/*2 (AA) homozygotes (n = 13), 21% *2/wild-type heterozygotes (AG) (n = 73), and 75% wild-type/wild-type homozygotes (GG) (n = 260). The genotype frequencies were consistent with Hardy-Weinberg predictions.
| Variable | Clopidogrel Low responders (VASP >50%) (n = 151) | Clopidogrel Responders (VASP <50%) (n = 195) | p Value |
|---|---|---|---|
| Age (years) | 64 ± 12 | 64 ± 12 | 0.89 |
| Women | 30 (20%) | 35 (18%) | 0.68 |
| Body mass index (kg/m 2 ) | 27.8 ± 6 | 26.5 ± 5 | 0.02 |
| Hypertension | 94 (62%) | 115 (59%) | 0.58 |
| Diabetes mellitus | 43 (28%) | 56 (29%) | 1.0 |
| Smoker | 66 (44%) | 73 (37%) | 0.27 |
| Dyslipidemia | 86 (57%) | 106 (54%) | 0.66 |
| History of coronary artery disease | 36 (24%) | 71 (36%) | 0.02 |
| β blockers | 114 (75%) | 150 (77%) | 0.80 |
| Angiotensin-converting enzyme inhibitors | 104 (69%) | 142 (73%) | 0.47 |
| Calcium antagonists | 14 (9%) | 18 (9%) | 1.0 |
| Proton pump inhibitors | 134 (89%) | 168 (86%) | 0.52 |
| Statin therapy | 141 (93%) | 183 (94%) | 1.0 |
| Left ventricular function | 54 ± 8 | 55 ± 7 | 0.80 |
| Creatinine (μmol/L) | 90 ± 49 | 90 ± 28 | 0.20 |
Clopidogrel response was assessed with PRI VASP, and we observed a high rate of low response in the whole population, with 41% of clopidogrel low responders after 600 mg (n = 151). Clopidogrel response was significantly affected by genotype, with lower clopidogrel response in CYP2C19*2 allele carriers (p = 0.01; Figure 1 ) . Accordingly, the rate of clopidogrel low response was higher in CYP2C19*2 allele carriers: 53% (n = 46 of 86) compared to 41% (n = 105 of 260) (p = 0.04). Among *2/*2 homozygotes (n = 13), 77% (n = 10) were nonresponders after 600-mg loading doses ( Figure 1 ). The mean response to clopidogrel in patients identified as low responders was not different between CYP2C19*2 allele carriers and noncarriers (66 ± 10% vs 67 ± 11%, p = 0.58; Figure 2 ) .
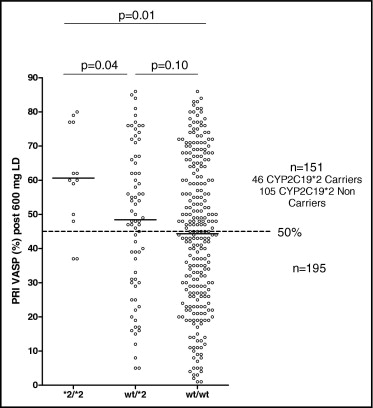
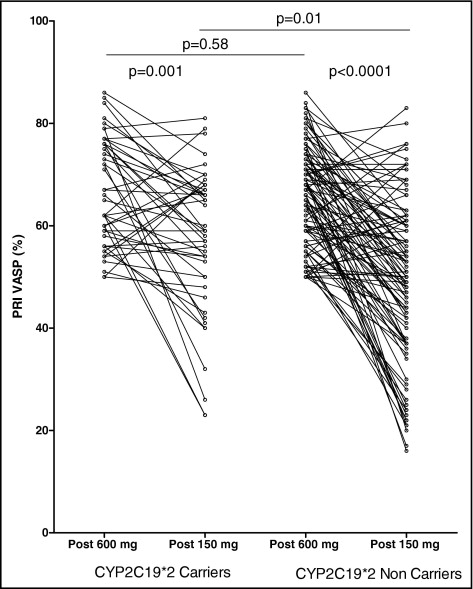
All clopidogrel low responders (n = 151), including 105 *2 noncarriers and 46 *2 carriers, received tailored therapy with high 150-mg clopidogrel maintenance at discharge, aiming to overcome initial low response. After 1 month, in the whole population of clopidogrel low responders, tailored therapy with a high 150-mg maintenance dose was able to overcome clopidogrel low response in only 44% of the patients (n = 66 of 151). The benefit of tailored therapy was significantly affected by CYP2C19 genotypes. Indeed, mean clopidogrel response after 1 month of tailored therapy was significantly lower in *2 carriers than in *2 noncarriers (57 ± 14% vs 50 ± 16%, p = 0.01; Figure 2 ). The mean variation of PRI VASP from the in-hospital phase to 1-month follow-up was significantly lower in CYP2C19*2 carriers (9.5% vs 15.7% of PRI VASP reduction, p <0.01; Figure 3 ) . In addition, initial low response was overcome by tailored therapy significantly less frequently in *2 carriers than in noncarriers (28% [n = 13 of 46] vs 50% [n = 53 of 105], p = 0.003; Figure 3 ). Among initial *2/*2 homozygotes with low response (n = 10), only 1 patient was a responder at 1 month, with 90% of patients (n = 9) still low responders.