Malnutrition is becoming one of the most important determinants of worse clinical outcomes in patients with acute heart failure (AHF). However, appropriate tools for evaluating the nutritional status in patients aged ≥65 years with AHF remain unclear. We examined 490 consecutive patients aged ≥65 years with AHF. They were divided into 2 groups according to Geriatric Nutritional Risk Index (GNRI; cut-off value = 92). During a median period of 189 days, the mortality rate was significantly higher in the lower GNRI group than the higher GNRI group (p <0.001). In multivariate analyses, lower GNRI was an independent determinant of adverse events (HR 0.92, 95% CI 0.88 to 0.95, p <0.001). The GNRI showed the best prognostic value (C-statistic: 0.70) among other nutritional indexes. Adding GNRI to an existing outcome prediction model for mortality in AHF significantly increased the C-statistic from 0.68 to 0.74 (p = 0.017). The net reclassification improvement afforded by GNRI was 60% overall, 27% for events, and 33% for nonevents (p <0.001). In conclusion, lower GNRI on admission was independently associated with worse clinical outcomes in patients aged ≥65 years with AHF, and it was superior to other nutritional parameters. Furthermore, the assessment of nutritional status using GNRI is very helpful for risk stratification.
The aim of the current investigation was first to examine the prognostic value of malnutrition assessed by the Geriatric Nutritional Risk Index (GNRI) on admission in patients aged ≥65 years with acute heart failure (AHF) and second to validate the clinical application of the index by comparing it with other nutritional markers and indexes, and by adding it to an existing outcome prediction model for AHF.
Methods
Data from the National cerebral and cardiovascular center acute Decompensated heart Failure (NaDEF) registry, which were obtained from January 2013 to March 2015, were retrospectively analyzed. The NaDEF registry is a Japanese single-center, observational, on-going, prospective cohort that includes all consecutive patients aged above 20 requiring hospitalization for the first time with a diagnosis of AHF by at least 2 experienced cardiologists according to the Framingham criteria, and follow-up was performed at 3, 6, 12, and 24 months after discharge by direct contact with patients or their physicians at the hospital or outpatient clinic, telephone interview of patients or, if deceased, of family members, and mail, by dedicated coordinators and investigators. In this study, because patient information was anonymized and deidentified before analyses, written informed consent was not obtained from each patient. However, we publicized the study by posting a summary of the protocol (with an easily understood description) on the website of the National Cerebral and Cardiovascular Center; the notice clearly informed patients of their right to refuse enrollment. These procedures for informed consent and enrollment are in accordance with the detailed regulations regarding informed consent described in the guidelines, and this study, including the procedure for enrollment, has been approved by the Institutional Review Board of the National Cerebral and Cardiovascular Center (M22-025) and registered under the Japanese UMIN Clinical Trials Registration (UMIN000017024).
From the 651 patients enrolled in the NaDEF registry, those with acute coronary syndrome or under 64 years old, or without GNRI data were excluded, and a total of 490 patients were ultimately enrolled in this study.
Because GNRI is calculated from serum albumin, actual and ideal body weight but not body mass index (BMI) in the original study by Bouillanne et al, we adopt their formula on admission as followings: GNRI = 1.489 × albumin (g/L) + 41.7 × (actual body weight/ideal body weight). When the patient’s weight exceeded the ideal weight, “(weight/ideal weight)” was set to 1. The GNRI formula results from the replacement of ideal weight in the Nutritional Risk Index (NRI) formula by usual weight as calculated using the Lorentz formula. GNRI cut-off values were also adopted from the report of Bouillanne et al. They defined 4 grades of nutrition-related risk: major risk (GNRI: <82), moderate risk (GNRI: 82 to <92), low risk (GNRI: 92 to <98), and no risk (GNRI: ≥98). In the present study, we defined the cut-off value of GNRI as 92.
We examined whether addition of nutritional assessment might improve the predictive ability of an existing prognostic model for AHF. As an existing representative prognostic model, the risk prediction nomogram from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) was used. The OPTIMIZE-HF trial is one of the largest hospital-based registries for new or worsening HF, and the risk prediction nomogram for all-cause death derived from this trial represents other similar risk models. Briefly, the nomogram is based on age, body weight, systolic blood pressure, serum sodium, and creatinine levels combined with baseline risk factors such as a history of liver disease, depression, and reactive airway disease.
The primary clinical outcome was all-cause death. Secondary outcomes were cardiovascular death and noncardiovascular death after initial nutritional assessment. Cardiovascular death was defined as death attributable to cardiovascular origin.
Results are presented as mean ± SD when normally distributed and as median and interquartile range when nonnormally distributed. Comparisons of differences between groups were made by unpaired Student t test or Mann–Whitney U test when appropriate. For survival analyses, Kaplan–Meier survival plots were constructed categorized by GNRI on admission into 2 groups, and log-rank testing was performed, to study the influence of GNRI on all-cause mortality. Cox proportional hazards regression was performed to estimate the effect of GNRI on all-cause mortality. Univariate factors that had a value of p <0.10 were identified. Finally, these factors were entered into the multivariate model to assess the prognostic value of GNRI. The prognostic value of GNRI was compared with that of BMI, serum albumin, and other nutritional markers, such as total cholesterol and lymphocyte count, using C-statistics. We added GNRI to the OPTIMIZE-HF nomogram and compared the C-statistics and the net reclassification improvement. All tests were 2 tailed, and a value of p <0.05 was considered statistically significant. All analyses were performed using JMP Pro 11 (SAS Institute Inc., Cary, North Carolina).
Results
During a median follow-up period of 189 days (interquartile range: 66 to 421 days), clinical outcomes occurred in 55 of study patients (11%), including 34 patients (7%) with cardiovascular death. Patients with lower GNRI (<92) had higher age, higher C-reactive protein and plasma B-type natriuretic peptide levels, and lower BMI, systolic blood pressure, hemoglobin, serum sodium, and serum albumin levels on admission than those with higher GNRI (≥92). There were no significant differences between the 2 groups in terms of gender, left ventricular ejection fraction, total cholesterol, peripheral lymphocyte count, and estimated glomerular filtration rate levels on admission ( Tables 1 and 2 ).
| Variable | Overall (n = 490) | GNRI | P-value | |
|---|---|---|---|---|
| < 92 (n = 162) | ≥ 92 (n = 328) | |||
| Age (years) | 79 ± 7 | 81 ± 8 | 78 ± 7 | < 0.001 |
| Male gender | 287 (59 %) | 94 (58 %) | 193 (59 %) | 0.92 |
| Body mass index (kg/m 2 ) | 22.6 ± 3.7 | 20.3 ± 3.5 | 23.7 ± 3.4 | < 0.001 |
| Hypertension | 360 (74 %) | 105 (65 %) | 255 (78 %) | 0.002 |
| Dyslipidemia | 260 (53 %) | 67 (41 %) | 193 (59 %) | < 0.001 |
| Diabetes mellitus | 180 (37 %) | 52 (32 %) | 128 (39 %) | 0.162 |
| Smoker | 252 (52 %) | 79 (49 %) | 173 (53 %) | 0.44 |
| Revascularization | 155 (32 %) | 46 (28 %) | 109 (33 %) | 0.30 |
| Chronic kidney disease | 304 (62 %) | 97 (60 %) | 207 (64 %) | 0.49 |
| Heart failure admission | 245 (50 %) | 94 (58 %) | 151 (46 %) | 0.016 |
| New York Heart Association class III or IV | 402 (89 %) | 129 (89 %) | 273 (89 %) | 1.00 |
| Heart rate (beats/min) | 90 ± 28 | 91 ± 27 | 90 ± 28 | 0.76 |
| Systolic blood pressure (mmHg) | 140 ± 31 | 134 ± 31 | 142 ± 31 | 0.010 |
| Left ventricular ejection fraction (%) | 40 (25 – 55) | 39 (24 – 54) | 40 (25 – 55) | 0.52 |
| Left ventricular diastolic diameter (mm) | 53 ± 10 | 52 ± 10 | 53 ± 10 | 0.61 |
| Etiology of heart failure | ||||
| Ischemic cardiomyopathy | 125 (26 %) | 45 (28 %) | 80 (24 %) | 0.44 |
| Non-ischemic cardiomyopathy | 150 (30 %) | 44 (27 %) | 106 (32 %) | 0.25 |
| Valvular disease | 131 (27 %) | 46 (28 %) | 85 (26 %) | 0.59 |
| Medications | ||||
| Aspirin | 178 (36 %) | 56 (35 %) | 122 (37 %) | 0.62 |
| Beta-blockers | 264 (54 %) | 88 (54 %) | 176 (54 %) | 0.92 |
| Statins | 188 (38 %) | 46 (28 %) | 142 (43 %) | 0.002 |
| Angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers | 269 (55 %) | 83 (51 %) | 186 (57 %) | 0.29 |
| Diuretics | 313 (64 %) | 114 (70 %) | 199 (61 %) | 0.036 |
| Variable | Overall (n = 490) | GNRI | P-value | |
|---|---|---|---|---|
| < 92 (n = 162) | ≥ 92 (n = 328) | |||
| Hemoglobin (g/dL) | 11.7 ± 2.1 | 11.2 ± 2.2 | 11.9 ± 2.0 | < 0.001 |
| Serum sodium (mEq/L) | 140 (138 – 142) | 139 (137 – 142) | 140 (138 – 143) | 0.017 |
| Total bilirubin (mg/dL) | 0.7 (0.5 – 1.0) | 0.7 (0.5 – 1.0) | 0.7 (0.5 – 1.0) | 0.66 |
| Estimated glomerular filtration rate (mL/min/1.73m 2 ) | 43 ± 20 | 43 ± 24 | 43 ± 18 | 0.98 |
| C-reactive protein (mg/dL) | 0.42 (0.13 – 1.26) | 0.61 (0.17 – 2.65) | 0.35 (0.11 – 0.91) | < 0.001 |
| Brain natriuretic peptide (pg/mL) | 583 (323 – 1080) | 696 (348 – 1434) | 551 (294 – 927) | < 0.001 |
| Lymphocyte count (/μL) | 1040 (706 – 1496) | 960 (657 – 1341) | 1109 (760 – 1619) | 0.069 |
| Albumin (g/dL) | 3.8 (3.4 – 4.0) | 3.3 (3.1 – 3.5) | 3.9 (3.7 – 4.1) | < 0.001 |
| Total cholesterol (mg/dL) | 149 (130 – 177) | 144 (121 – 176) | 154 (133 – 179) | 0.052 |
| Geriatric nutritional risk index | 98.2 ± 10.4 | 86.1 ± 4.5 | 99.2 ± 4.8 | < 0.001 |
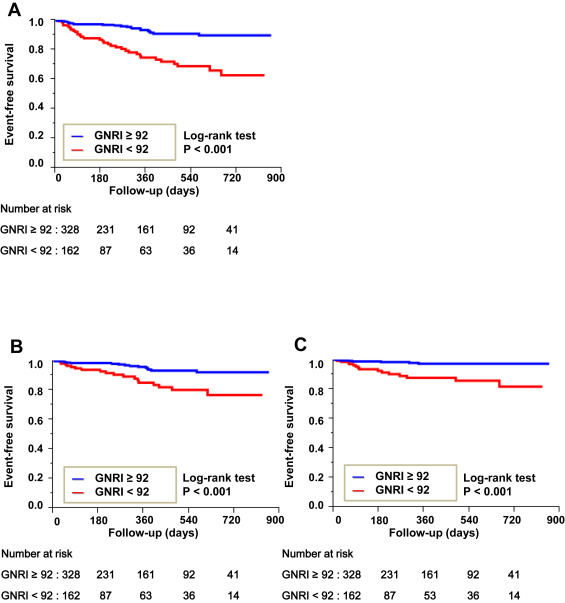
Kaplan–Meier survival curves revealed that lower GNRI was associated with a higher incidence of all-cause death, cardiovascular death, and noncardiovascular death during the observation period ( Figure 1 ).
Univariate Cox proportional hazards model analysis for the determinants of all-cause mortality showed that GNRI, a history of HF admission, systolic blood pressure, hemoglobin, serum sodium, estimated glomerular filtration rate, lymphocyte count, and total cholesterol levels were variables with p values <0.10. The other examined variables, including age, log B-type natriuretic peptide and left ventricular ejection fraction, were not significant. In multivariate Cox proportional hazards model analyses, lower GNRI was confirmed to be an independent determinant of increased all-cause mortality ( Table 3 ).
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Geriatric nutritional risk index | 0.90 | 0.87 – 0.94 | < 0.001 | 0.92 | 0.88 – 0.95 | < 0.001 |
| Age | 1.01 | 0.98 – 1.05 | 0.51 | – | – | – |
| History of heart failure admission | 2.38 | 1.35 – 4.38 | 0.002 | 2.11 | 1.10 – 4.19 | 0.024 |
| Hypertension | 0.83 | 0.48 – 1.50 | 0.52 | – | – | – |
| Dyslipidemia | 0.81 | 0.48 – 1.38 | 0.43 | – | – | – |
| Diabetes mellitus | 0.90 | 0.50 – 1.56 | 0.73 | – | – | – |
| Systolic blood pressure | 0.98 | 0.97 – 0.99 | < 0.001 | 0.99 | 0.97 – 1.00 | 0.025 |
| Use of statins | 0.67 | 0.38 – 1.17 | 0.161 | – | – | – |
| Hemoglobin | 0.85 | 0.75 – 0.97 | 0.019 | 0.97 | 0.82 – 1.13 | 0.66 |
| Serum sodium | 0.93 | 0.89 – 0.98 | 0.009 | 0.96 | 0.90 – 1.03 | 0.22 |
| Log brain natriuretic peptide | 1.12 | 0.84 – 1.52 | 0.44 | – | – | – |
| Estimated glomerular filtration rate | 0.98 | 0.97 – 0.99 | < 0.001 | 1.00 | 0.98 – 1.02 | 0.95 |
| Lymphocyte count | 1.00 | 1.00 – 1.00 | < 0.001 | 0.99 | 0.95 – 1.02 | 0.48 |
| Total cholesterol | 0.99 | 0.98 – 1.00 | 0.012 | 1.00 | 0.99 – 1.01 | 0.49 |
| Left ventricular ejection fraction | 0.99 | 0.97 – 1.01 | 0.33 | – | – | – |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


