We aimed to evaluate the prevalence and prognostic role of fragmented QRS complex (fQRS) in predicting arrhythmic events and cardiovascular mortality in patients with noncompaction cardiomyopathy (NCC). A total of 88 patients (64.8% men, mean age 38.6 ± 17.7 years) with the diagnosis of NCC were enrolled. Median follow-up time was 42.4 months. The fQRS was defined as the presence of ≥1 additional R wave (R′) or notch on the R/S waves in ≥2 contiguous leads representing anterior (V1 to V5), inferior (II, III, and aVF), or lateral (I, aVL, and V6) myocardial segments. Compared to patients without fQRS group, patients with fQRS (fQRS (+) group) showed higher rates for total arrhythmic events, ventricular tachycardia, bradyarrhythmia requiring pacemaker, sudden cardiac death, cardiovascular mortality, and all-cause mortality. The cut-off point of ≥3 leads for the fQRS was the optimal point discriminating an arrhythmic event and cardiovascular mortality. In Kaplan–Meier survival analysis, total arrhythmic events and cardiovascular mortality occurred more frequently in the fQRS (+) group. In multivariate Cox proportional hazard regression analysis, after adjusting for other confounding factors, the presence of fQRS were found to be as an independent predictor of arrhythmic events (hazard ratio 3.850, 95% CI 1.062 to 9.947, p = 0.002) and cardiovascular mortality (hazard ratio 2.719, 95% CI 1.494 to 9.262, p = 0.005). In conclusion, the presence of fQRS complex, as a simple and feasible electrocardiographic marker, seems to be a novel predictor of arrhythmic events and cardiovascular mortality in patients with NCC. This simple parameter may be used in identifying patients at high risk for arrhythmic events and so individualization of specific therapies can be applied.
Noncompaction cardiomyopathy (NCC), as a new and yet unclassified cardiomyopathy, is characterized by specific morphologic abnormalities in ventricular myocardium, including prominent trabeculations, deep intertrabecular recesses, and thin compacted layer. Clinical picture range from asymptomatic status to heart failure, systemic thromboembolic events, and life-threatening arrhythmias such as ventricular fibrillation (VF), ventricular tachycardia (VT), atrioventricular block, and even sudden cardiac death (SCD). Electrocardiographic (ECG) changes and clinical arrhythmias may occur as an initial presentation and play a crucial role in the course of the disease. Fragmented QRS complex (fQRS) on a routine 12-lead electrocardiogram, as a marker of depolarization abnormality, represents the conduction delay in myocardial activation because of myocardial scarring and suggested to be a novel indicator of mortality and malignant arrhythmic events in various cardiovascular diseases. However, there are scarce data on the prognostic role of fQRS in cardiac arrhythmias and mortality in NCC. Therefore, we aimed to evaluate the prognostic role of fQRS in predicting arrhythmic events and cardiovascular mortality in patients with NCC.
Methods
We performed a retrospective analysis of a prospectively defined cohort of 88 consecutive patients (64.8% men, mean age 38.6 ± 17.7 years) diagnosed with NCC in our tertiary, heart-specialized hospital from April 2004 to April 2015. Demographic, ECG, and echocardiographic data of all patients were collected from clinical follow-up visits, patients’ files, and the electronic database. Informed consent was taken from each patient before enrollment. The study was in compliance with the principles outlined in the Declaration of Helsinki and approved by institutional ethics committee.
The fQRS was defined based on previously described criteria as follows: in patients with narrow QRS complexes, (1) an additional R wave (R′), (2) notching in the nadir of the S wave, (3) notching of the R wave, or (4) the presence of more than one R′ ( Figure 1 ); in patients with wide QRS complexes (QRS duration >120 ms), (1) various RsR′ patterns with >2 R′, (2) >2 notches in the R wave, or (3) >2 notches in the downstroke or upstroke of the S wave. In addition, fragmentation should be detected in ≥2 contiguous leads representing anterior (V1 to V5), inferior (II, III, and aVF), or lateral (I, aVL, and V6) myocardial segments. The extent of fQRS in each patient was assessed by counting the number of ECG leads with fQRS. In patients with fQRS, the number of spikes in fQRS configuration was counted in the lead containing the maximal number of spikes.
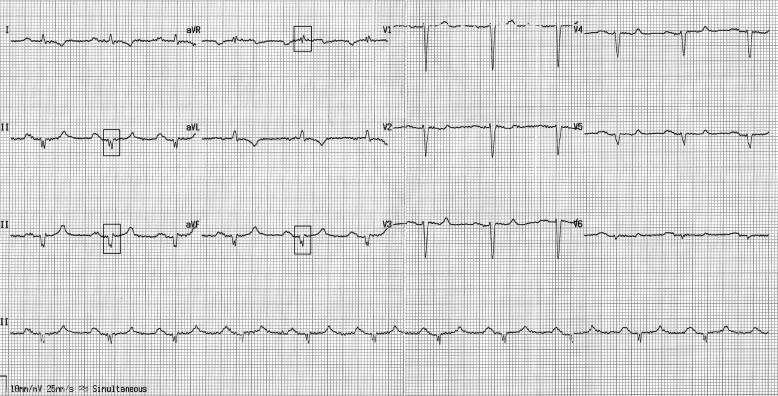
The 12-lead electrocardiogram of each patient was assessed by 2 independent cardiologists blinded to the patients’ clinical outcomes. The intraobserver and interobserver reliabilities for detecting the presence of fQRS were found to be as κ= 0.977 (p <0.001) and 0.932 (p <0.001), respectively. All patients had repeated electrocardiograms during follow-up. We determined the κvalue for intraobserver reproducibility as 0.909 (p <0.001).
The 2-dimension and Doppler transthoracic echocardiography examinations were performed by experienced echocardiographers using 2.5 to 4-MHz transducers (Vivid 7; GE Medical System, Milwaukee, Wisconsin). The NCC was diagnosed in regards to the following proposed criteria: (1) characteristic 2-layered myocardium with a compacted epicardial layer and a thicker noncompacted endocardial layer. The suggested ratio of noncompacted to compacted layer measured at end-systolic phase is >2. (2) Characteristic segmental localization of the noncompacted wall is apical and midventricular parts of the inferior and lateral walls. (3) Filling of the intertrabecular recesses with ventricular blood using color Doppler imaging. The noncompacted to compacted ratio was also evaluated.
An implantable cardioverter defibrillator (ICD) or a cardiac resynchronization therapy device has been implanted for primary or secondary prevention purpose according to the contemporary updated device guidelines.
The follow-up duration was initiated with the first visit and ended with the occurrence of death, arrhythmic event(s), or the last visit. The primary end point was the occurrence of an arrhythmic event (a malignant cardiac arrhythmia [sustained VT and/or VF and/or appropriate ICD shock], bradyarrhythmia requiring pacemaker implantation and SCD) and cardiovascular mortality. Patients who initially presented with arrhythmic event or SCD and experienced only inappropriate shocks have been excluded from the analysis. Follow-up visits including clinical assessment and electrocardiogram were regularly performed at 3-to 6-month intervals. The ICD devices were routinely interrogated when there is any relevant symptom and also at 6-month intervals. Stored intracardiac ECG recordings were evaluated in terms of cardiac arrhythmias.
Besides periodic clinical follow-up in the hospital, the follow-up data were documented from the hospital records, phone calls to the patients, their relatives, and/or their general practitioners, as well as from the pharmacy database. The cause of death was determined based on the hospital records and death certificates obtained from the Turkey Ministry of Health, Turkish National Population Register.
Statistical analyses were performed using SPSS statistical software version 20.0 (SPSS Inc., Chicago, Illinois). Continuous variables are represented as mean ± S.D, and categorical variables are reported as percentage. The Kolmogorov–Smirnov criterion was used for the assessment of normality. Comparisons of continuous variables between the 2 groups were analyzed with the independent samples t test, and the chi-square test was used for categorical variables. Intraobserver and interobserver reliability analyses using the Kappa statistic were applied to assess the consistency in determination of fQRS. The univariate and multivariate Cox proportional hazard regression analyses were performed to evaluate the association of the variables with the occurrence of arrhythmic events and cardiovascular mortality, respectively. The Kaplan–Meier curve analysis was used for freedom from arrhythmic events and survival in patients with or without fQRS. Receiver operating characteristic curve analysis was used to determine the optimum cut-off levels of the number of derivations with fQRS to predict the arrhythmic events and mortality. A p value <0.05 was considered statistically significant.
Results
Baseline demographical, clinical, ECG, and echocardiographic characteristics of the study population were presented in Table 1 . Dyspnea was the most common clinical presentation, noted in 52 patients (59.1%). VT (n = 14, 15.9%), syncope (n = 9, 12.3%), and aborted SCD (n = 7, 8.0%) were also reported on admission. A total of 47 patients (53.4%) revealed fQRS in ≥2 contiguous leads. QRS fragmentation was primarily localized at inferior (38 patients) and precordial (31 patients) leads and predominantly demonstrated in the R wave (36 patients). Although patients in fQRS (+) group revealed lower left ventricular ejection fraction (LVEF), the ratio of noncompacted to compacted myocardium was significantly greater compared to fQRS (−) group (p <0.05).
| Variables | All patients (n=88) | fQRS (+) (n=47) | fQRS (-) (n=41) | P Value |
|---|---|---|---|---|
| Age (years) | 38.6±17.7 | 38.2±17.9 | 39.2±17.9 | 0.840 |
| Male gender | 57 (64.8%) | 30 (63.8%) | 27(65.9%) | 0.511 |
| Hypertension | 15 (17%) | 9 (19.1%) | 6 (14.6%) | 0.393 |
| Hyperlipidemia | 13 (14.8%) | 7 (14.9%) | 6 (14.6%) | 0.973 |
| Smoking | 13 (14.8%) | 6 (12.8%) | 7 (17.1%) | 0.570 |
| Diabetes mellitus | 8 (9.1%) | 5 (10.6%) | 3 (7.3%) | 0.589 |
| BMI (kg/m 2 ) | 22.9±4.3 | 22.8±4.9 | 23.0±3.6 | 0.912 |
| Coronary artery disease | 12 (13.6%) | 6 (12.8%) | 6 (14.6%) | 0.799 |
| ICD/CRT-D implantation | 55 (62.5%) | 31 (66.0%) | 24 (58.5%) | 0.473 |
| CRT-D implantation | 19 (21.6%) | 14 (29.8%) | 5 (12.2%) | 0.039 |
| Clinical presentation | ||||
| Dyspnea | 52 (59.1%) | 22 (53.7%) | 30 (63.8%) | 0.333 |
| Chest pain | 16 (18.4%) | 9 (19.1%) | 7 (17.5%) | 0.843 |
| Palpitation | 17 (19.5%) | 12 (25.5%) | 5 (12.5%) | 0.127 |
| Syncope | 9 (12.3%) | 7 (14.9%) | 2 (4.9%) | 0.122 |
| Ventricular tachycardia | 14 (15.9%) | 9 (19.1%) | 5 (12.2%) | 0.374 |
| Aborted sudden cardiac death | 7 (8.0%) | 6 (12.8%) | 1 (2.4%) | 0.074 |
| Thromboembolic events | 6 (6.8%) | 4 (8.5%) | 2 (4.9%) | 0.500 |
| Functional Status | ||||
| NYHA class I-II | 59 (77.1%) | 28 (59.5%) | 31 (75.6%) | 0.110 |
| NYHA class III-IV | 29 (32.9%) | 19 (40.4%) | 10 (24.4%) | 0.110 |
| Family history of sudden cardiac death | 14 (15.9%) | 8 (17.0%) | 6 (14.6%) | 0.760 |
| Family history of NCC | 5 (5.7%) | 3 (6.4%) | 2 (4.9%) | 0.761 |
| Medical treatment | ||||
| Beta blockers | 66 (75.0%) | 36 (76.6%) | 30 (73.2%) | 0.711 |
| ACE inhibitor or ARB | 60 (72.3%) | 34 (72.3%) | 26 (63.4%) | 0.370 |
| Spironolactone | 41 (46.6%) | 23 (48.9%) | 18 (43.9%) | 0.637 |
| Diuretics | 54 (61.4%) | 30 (63.8%) | 24 (58.5%) | 0.611 |
| Amiodarone | 6 (6.8%) | 5 (10.6%) | 1 (2.4%) | 0.128 |
| Sotalol | 3 (3.4%) | 2 (4.3%) | 1 (2.4%) | 0.640 |
| 24-hour holter findings | ||||
| Frequent VPB | 40 (45.5%) | 28 (59.6%) | 12 (29.3%) | 0.004 |
| Bradycardia | 14 (15.9%) | 10 (21.3%) | 4 (9.8%) | 0.141 |
| Nonsustained VT | 45 (51.1%) | 29 (61.7%) | 15 (36.6%) | 0.019 |
| Electrocardiographic parameters | ||||
| Atrial fibrillation | 17 (19.3%) | 13 (27.7%) | 4 (9.8%) | 0.034 |
| Duration of PR interval | 156.3±48.9 | 158.5±49.3 | 153.8±49.0 | 0.676 |
| Duration of QRS interval | 108.9±37.3 | 112±39.5 | 102.0±33.8 | 0.105 |
| Duration of QTc interval | 421.0±46.2 | 426.2±48.7 | 415±42.8 | 0.265 |
| RBBB | 6 (6.8%) | 5 (10.6%) | 1 (2.4%) | 0.128 |
| LBBB | 9 (10.3%) | 8 (17.4%) | 1 (2.4%) | 0.022 |
| Echocardiographic parameters | ||||
| LVEDD (mm) | 59.3±9.1 | 56.3±8.8 | 62.3±8.6 | 0.043 |
| LVESD (mm) | 48.4±11.9 | 52.8±10.6 | 44.1±11.8 | 0.025 |
| LA diameter (mm) | 42.7±10.2 | 47.1±9.8 | 38.3±10.7 | 0.008 |
| LVEF (%) | 32.0±12.5 | 27.5±11.7 | 36.9±14.3 | 0.006 |
| Non-compact/compact ratio | 2.6±0.3 | 2.7±0.3 | 2.5±0.2 | 0.004 |
Although there was a significant correlation between the noncompacted/compacted ratio and extent of fQRS (p <0.001), the r value was relatively weak (Spearman’s rho = 0.477).
Follow-up data and clinical outcomes were presented in Table 2 . The fQRS (+) revealed higher rates of total arrhythmic events, sustained VTs, bradyarrhythmia requiring pacemaker implantation, and SCD. Cardiovascular and all-cause mortality were also significantly greater in patients with fQRS (p <0.05). During median 42.4 (range: 1.1 to 120.3) months of follow-up, a total of 55 patients (62.5%) underwent ICD or cardiac resynchronization therapy device implantation (primary prophylaxis for 36 patients and secondary prophylaxis for 18 patients). In Kaplan–Meier survival analysis, total arrhythmic events occurred more frequently in the fQRS (+) group (log-rank, p = 0.005, Figure 2 ). Also, patients with fQRS had a higher cardiovascular mortality rate compared with patients without fQRS (log-rank, p = 0.004, Figure 3 ).
| Parameter | All patients (n=88) | fQRS (+) (n=47) | fQRS (-) (n=41) | p Value |
|---|---|---|---|---|
| Total arrhythmic events | 50(56.8%) | 34(72.3%) | 16(39%) | 0.002 |
| Sustained VT/VF | 47(53.4%) | 31(66.0%) | 16(39.0%) | 0.012 |
| Bradyarrhythmia requiring PM implantation | 8(14.9%) | 7(14.9%) | 1(2.4%) | 0.043 |
| Sudden cardiac death | 9(10.2%) | 8(17.0%) | 1(2.4%) | 0.024 |
| Cardiovascular death | 27(31%) | 21(45.7%) | 6(14.6%) | 0.002 |
| All-cause death | 32(36.8%) | 24(52.2%) | 8(19.5%) | 0.002 |
In receiver operating characteristic analysis, a cut-off point of ≥3 leads for the fQRS was determined to be an optimal point in discriminating arrhythmic events (sensitivity 52%, specificity 90.5%, area under curve [AUC] = 0.716, p = 0.001) and all-cause mortality (sensitivity 55.6%, specificity 78.7%, AUC = 0.680, p = 0.008; Figure 4 ). Also, the cut-off point of ≥3 spikes in the most fragmented QRS was found to have 61.6% sensitivity, 91.6% specificity in predicting arrhythmic events (AUC = 0.738, p <0.001), and all-cause mortality (sensitivity 60.7%, specificity 84.2%, AUC = 0.724, p <0.001).
In multivariate Cox regression analysis, the presence of fQRS was determined as an independent predictor of arrhythmic events (hazard ratio 3.85, 95% CI 1.06 to 9.95, p = 0.002) even after adjusting for other confounding factors ( Table 3 ). In addition, the presence of fQRS (hazard ratio 2.719, 95% CI 1.494 to 9.262, p = 0.005) was also found as an independent predictor of cardiovascular mortality ( Table 4 ).
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95 % CI | P Value | Adjusted OR | 95 % CI | p value | |
| Age | 0.991 | 0.965-1.017 | 0.476 | |||
| Male gender | 1.057 | 0.514-2.174 | 0.880 | |||
| Hypertension | 0.728 | 0.280-1.894 | 0.516 | |||
| Hyperlipidemia | 0.366 | 0.087-1.534 | 0.169 | |||
| Smoking | 0.483 | 0.147-1.591 | 0.232 | |||
| Diabetes mellitus | 1.090 | 0.378-3.143 | 0.873 | |||
| BMI | 0.761 | 0.991-1.020 | 0.298 | |||
| Coronary artery disease | 0.510 | 0.155-1.675 | 0.267 | |||
| Clinical presentation | ||||||
| Syncope | 0.859 | 0.727-1.015 | 0.104 | |||
| VT | 3.024 | 1.004-9.044 | 0.009 | 2.609 | 1.361-9.715 | 0.003 |
| Aborted SCD | 3.494 | 1.599-12.628 | <0.001 | 2.824 | 1.654-8.883 | <0.001 |
| Tromboembolic events | 1.485 | 0.793-2.781 | 0.217 | |||
| NYHA class III-IV | 1.285 | 0.834-1.981 | 0.256 | |||
| Family history of SCD | 2.442 | 2.213-6.921 | 0.009 | 1.948 | 1.407-4.651 | 0.007 |
| Family history of NCC | 2.741 | 0.433-17.358 | 0.284 | |||
| Medical treatment | ||||||
| Beta blocker | 0.638 | 0.184-2.209 | 0.478 | |||
| ACE inhibitor or ARB | 1.715 | 0.478-6.161 | 0.408 | |||
| Spironolactone | 0.659 | 0.254-1.709 | 0.391 | |||
| Diuretics | 0.978 | 0.320-2.983 | 0.968 | |||
| Amiodarone | 1.045 | 0.274-3.985 | 0.949 | |||
| Sotalol | 2.014 | 0.564-7.193 | 0.281 | |||
| 24-hour holter findings | ||||||
| Frequent VPB, n (%) | 3.924 | 1.561-9.863 | 0.004 | 1.659 | 1.220-2.083 | 0.007 |
| Bradicardia, n (%) | 1.356 | 0.424-4.333 | 0.608 | |||
| Nonsustained VT, n (%) | 3.472 | 1.736-11.522 | 0.001 | 2.441 | 1.854-3.887 | 0.001 |
| ECG parameters | ||||||
| Atrial fibrillation | 1.182 | 1.077-1.616 | 0.012 | 1.168 | 0.852-1.783 | 0.606 |
| Duration of PR interval | 1.196 | 0.801-1.786 | 0.383 | |||
| Duration of QRS interval | 1.050 | 0.760-1.450 | 0.769 | |||
| Durarion of QTc interval | 1.305 | 1.186-1.917 | 0.018 | 1.712 | 0.825-2.611 | 0.192 |
| RBBB | 1.112 | 0.834-1.981 | 0.227 | |||
| LBBB | 1.661 | 1.143-2.728 | 0.004 | 1.338 | 1.104-2.455 | 0.017 |
| fQRS | 4.500 | 1.714-11.812 | 0.002 | 3.850 | 1.062-9.947 | 0.002 |
| Echocardiographic parameters | ||||||
| LVEDD (mm) | 1.291 | 1.013-1.531 | 0.008 | 1.166 | 1.093-1.805 | 0.021 |
| LVESD (mm) | 1.128 | 1.024-1.244 | 0.064 | |||
| LA (mm) | 1.074 | 0.998-1.157 | 0.057 | |||
| LVEF (%) | 2.164 | 1.148-3.237 | 0.001 | 1.717 | 1.306-2.380 | 0.001 |
| Non-compacted/compacted ratio | 1.613 | 1.246-2.455 | 0.009 | 1.205 | 1.136-1.277 | 0.015 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


