The Seattle Heart Failure Model (SHFM) is a validated prediction model that estimates the mortality in patients with chronic heart failure (CHF) using commonly obtained information, including clinical data, laboratory test results, medication use, and device implantation. In addition, cardiac iodine-123 meta-iodobenzylguanidine (MIBG) imaging provides prognostic information for patients with CHF. However, the long-term predictive value of combining the SHFM and cardiac MIBG imaging in patients with CHF has not been elucidated. To prospectively investigate whether cardiac iodine-123 MIBG imaging provides additional prognostic value to the SHFM in patients with CHF, we studied 106 outpatients with CHF who had radionuclide left ventricular ejection fraction <40% (30 ± 8%). The SHFM score was obtained at enrollment, and the cardiac MIBG washout rate (WR) was calculated from anterior chest images obtained at 20 and 200 minutes after isotope injection. During a mean follow-up of 6.8 ± 3.5 years (range 0 to 13), 32 of 106 patients died from cardiac causes. A multivariate Cox analysis revealed that the WR (p = 0.0002) and SHFM score (p = 0.0091) were independent predictors of cardiac death. Kaplan-Meier analysis showed that patients with an abnormal WR (>27%) had a significantly greater risk of cardiac death than did those with a normal WR for both those with a SHFM score of ≥1 (relative risk 3.3, 95% confidence interval 1.2 to 9.7, p = 0.01) and a SHFM score of ≤0 (relative risk 3.4, 95% confidence interval 1.2 to 9.6, p = 0.004). In conclusion, the cardiac MIBG WR provided additional prognostic information to the SHFM score for patients with CHF.
Despite advances in pharmacologic treatment, the mortality of patients with chronic heart failure (CHF) has remained high. The prediction of poor outcomes in patients with CHF can help physicians to guide therapy. Physicians need to counsel patients about their prognosis to enable informed decisions about medications, devices, transplantation, and end-of-life care. The Seattle Heart Failure Model (SHFM) is a validated prediction model that estimates total mortality for patients with CHF using commonly obtained clinical, laboratory, medication, and device variables. In addition, cardiac iodine-123 (I-123) meta-iodobenzylguanidine (MIBG) imaging, which is useful for the estimation of cardiac adrenergic nerve activity, has been reported to predict poor clinical outcomes in CHF. However, the long-term predictive value of the combination of SHFM and cardiac MIBG imaging in patients with CHF remains to be elucidated. The purpose of the present study was to prospectively investigate whether cardiac MIBG imaging can provide additional prognostic power to the SHFM for patients with CHF.
Methods
We enrolled 106 consecutive outpatients with CHF whose left ventricular ejection fraction (LVEF), measured by radionuclide angiography, was <40%. CHF was diagnosed from the clinical signs and symptoms according to the Framingham criteria. To be included in the present study, all patients who had experienced at least one episode of decompensated heart failure were required to be stable for ≥3 months on conventional therapy with angiotensin-converting enzyme inhibitors, diuretics, and digoxin. Patients were excluded from the present study if they had significant renal dysfunction, insulin-dependent diabetes mellitus, or autonomic neuropathy. None of the patients were receiving β blockers or had had an implantable cardioverter-defibrillator (ICD), biventricular pacemaker, or biventricular defibrillator at enrollment.
The mean patient age was 64 ± 12 years. Of the 106 patients, 81 were men and 25 were women. CHF was due to ischemic heart disease in 55 patients and idiopathic dilated cardiomyopathy in 51. The average New York Heart Association (NYHA) functional class was 2.1 ± 0.6, with 16% of patients having class I, 62% class II, and 22% class III. The LVEF was 30 ± 8%. At entry, all patients underwent cardiac MIBG imaging and echocardiography, and a venous blood sample was drawn. All patients gave written informed consent for their participation in the present study, which was approved by the Osaka General Medical Center’s Review Committee.
The development of the SHFM (available at: SeattleHeartFailureModel.org ) has been previously described. In brief, the model was derived from data from a single CHF population and validated using 5 additional CHF populations. Commonly obtained clinical variables (e.g., age, gender, NYHA class, medications, and laboratory values such as sodium, hemoglobin, and cholesterol) were evaluated as predictors of mortality, transformed as appropriate for nonlinear relations, and selected for final inclusion by stepwise forward selection in a multivariate Cox proportional hazards analysis. The hazard ratios for heart failure medications and devices were also estimated. To calculate the SHFM score for each patient, each variable in the model was multiplied by its β coefficient (natural log of the hazard ratio), and the products were summed. β-Blocker therapy (carvedilol) was initiated in 48 of the 106 study patients just after enrollment. In these patients, the SHFM score was calculated with the β blocker present.
All patients underwent myocardial imaging with I-123 MIBG (Fiji Film RI Pharma Laboratory, Tokyo, Japan) in the supine position using the same γ camera as that used for radionuclide angiography. No patients underwent withdrawal of β blockers before imaging. A 111-MBq dose of I-123 MIBG was injected intravenously with the patient at rest after an overnight fast. Initial and delayed image acquisitions were performed in the anterior chest view at 20 and 200 minutes after isotope injection, respectively. As previously described, 2 independent observers, who were unaware of the clinical status of the patients, assessed the cardiac MIBG uptake ( Figure 1 ). The heart/mediastinum ratio was determined by dividing the counts/pixel in a visually drawn heart region of interest by the counts/pixel in a 7 × 7 pixel upper mediastinum region of interest. After taking the radioactive decay of I-123 into consideration, the cardiac MIBG washout rate (WR) was calculated from the initial and delayed images. From the findings from our previous study, an abnormal WR was defined as >27%, which was the mean control WR ± 2 SD.
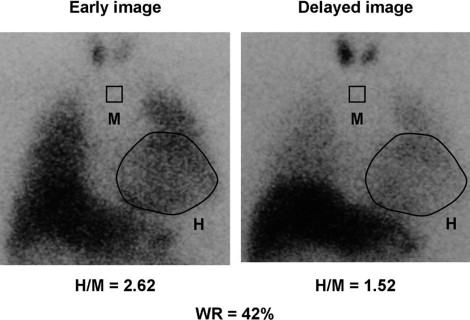
Patients underwent 24-hour electrocardiographic recording with a Marquette Electronics 8000 Holter monitoring system (Marquette Electronics, Milwaukee, Wisconsin). Ventricular arrhythmias were classified according to Lown’s grade, and nonsustained ventricular tachycardia was defined as ≥5 consecutive premature ventricular beats lasting <30 seconds. Furthermore, 2-dimensional echocardiography was performed using a Toshiba SSH-380A recorder equipped with a 2.5- or 3.75-MHz transducer. The left ventricular end-diastolic dimension and left atrial dimension were measured using standard techniques.
Blood sampling for assessment of the plasma noradrenaline concentration, serum sodium, creatinine, total cholesterol and uric acid levels, and hemoglobin was done from an intravenous cannula after the patients had rested for ≥30 minutes in the supine position. The plasma noradrenaline concentration was determined using high-performance liquid chromatography at Shionogi Biomedical Laboratories (Osaka, Japan). In 58 of 106 patients, the percentage of lymphocytes was not measured at entry, and the values were estimated from the total white blood cell counts to obtain the SHFM score.
All the study patients were followed up prospectively in our hospital at least once a month by clinicians who were not aware of the SHFM score or the results of cardiac MIBG imaging. The primary end point of the present study was cardiac death, which included sudden death (i.e., witnessed cardiac arrest, death within 1 hour after the onset of acute symptoms, or unexpected, unwitnessed death in a patient known to have been well within the previous 24 hours), pump failure death (i.e., progressively reduced cardiac output and failure of organ perfusion), or other cardiac death (e.g., acute myocardial infarction).
The data are presented as the mean ± SD. Student’s t test and Fisher’s exact test were used to compare the differences between the groups for the continuous and discrete variables, respectively. A Cox proportional hazards regression model was used to assess the association of the following baseline patient characteristics with survival: heart rate, presence of nonsustained ventricular tachycardia on Holter monitoring, echocardiographic data, plasma noradrenaline concentration, serum creatinine level, the results of cardiac MIBG imaging, and the SHFM score. The forward stepwise method was used for the multivariate analyses, with the entry and removal p values set at 0.05. The Kaplan-Meier method was used to calculate the event-free survival rate in the patients with a normal and abnormal WR, and the 2 groups were compared using a log-rank test. All statistical analyses were performed using StatView, version 5 (SAS Institute, Cary, North Carolina). p Values <0.05 were considered statistically significant.
Results
During a mean follow-up of 6.8 ± 3.5 years (range 0 to 13), 42 patients died. A cardiac cause was noted for 32 patients. Sudden death was the most common cause of cardiac death (n = 20), followed by pump failure death (n = 11) and death from acute myocardial infarction (n = 1).
The baseline characteristics of the patients with and without cardiac death are listed in Table 1 . No differences were found in age, gender, weight, NYHA class, proportion of ischemic heart disease, heart rate, blood pressure, drug use, serum sodium, creatinine and total cholesterol levels, hemoglobin, estimated percentage of lymphocytes, the presence of nonsustained ventricular tachycardia, or left atrial dimension between patients with and without cardiac death. Patients with cardiac death had a significantly lower LVEF, greater Lown’s grade and left ventricular end-diastolic dimension, and higher plasma noradrenaline and uric acid concentrations.
| Characteristic | Cardiac Death | p Value | |
|---|---|---|---|
| Yes (n = 32) | No (n = 74) | ||
| Demographics | |||
| Age (years) | 64 ± 11 | 63 ± 12 | 0.5592 |
| Men | 27 (84%) | 54 (73%) | 0.2080 |
| Weight (kg) | 61 ± 11 | 60 ± 12 | 0.6375 |
| Heart failure characteristics | |||
| New York Heart Association class | 2.2 ± 0.7 | 2.0 ± 0.6 | 0.1792 |
| Ischemia | 15 (47%) | 40 (54%) | 0.5017 |
| Heart rate (beats/min) | 72 ± 10 | 74 ± 12 | 0.3099 |
| Systolic pressure (mm Hg) | 124 ± 16 | 129 ± 18 | 0.1527 |
| Medications | |||
| β Blocker ⁎ | 11 (34%) | 37 (50%) | 0.2943 |
| Diuretics | 23 (72%) | 41 (56%) | 0.0835 |
| Angiotensin-converting enzyme inhibitors | 23 (72%) | 58 (78%) | 0.4738 |
| Angiotensin II receptor blockers | 1 (3%) | 9 (12%) | 0.1467 |
| Statins | 7 (22%) | 23 (31%) | 0.3388 |
| Allopurinol | 7 (22%) | 10 (14%) | 0.2859 |
| Aldosterone blocker | 21 (66%) | 48 (65%) | 0.9406 |
| Radionuclide angiography | |||
| Left ventricular ejection fraction (%) | 27 ± 8 | 31 ± 7 | 0.0054 |
| Holter electrocardiography | |||
| Lown’s grade | 3.86 ± 1.08 | 3.23 ± 1.51 | 0.0364 |
| Ventricular tachycardia (%) | 7 (22%) | 10 (14%) | 0.2997 |
| Echocardiography | |||
| Left ventricular end-diastolic dimension (mm) | 65 ± 8 | 62 ± 8 | 0.0387 |
| Left atrial dimension (mm) | 45 ± 7 | 42 ± 8 | 0.0703 |
| Laboratory findings | |||
| Noradrenaline (pg/ml) | 517 ± 240 | 416 ± 239 | 0.0499 |
| Sodium (mmol/L) | 138.3 ± 3.4 | 139.3 ± 3.0 | 0.1188 |
| Creatinine (mg/dl) | 0.99 ± 0.31 | 0.89 ± 0.24 | 0.0732 |
| Total cholesterol (mg/dl) | 199 ± 50 | 189 ± 41 | 0.2815 |
| Uric acid (mg/dl) | 7.5 ± 2.0 | 6.5 ± 1.9 | 0.0210 |
| Hemoglobin (g/dl) | 13.5 ± 1.5 | 13.4 ± 1.5 | 0.7323 |
| Estimated lymphocytes (%) | 26 ± 11 | 29 ± 10 | 0.4022 |
⁎ Use of β blocker (carvedilol) as scored just after enrollment.
The results of SHFM scoring and MIBG imaging are listed in Table 2 . The SHFM score was significantly greater in patients with than without cardiac death. Patients with a SHFM score of ≥1 (rounded data) had a significantly greater incidence of cardiac death than those with a SHFM score of ≤0 (47% vs 23%, respectively, p = 0.014). Furthermore, patients with cardiac death had a significantly lower heart-to-mediastinum ratio in the early and delayed images and a significantly greater WR than those without cardiac death ( Figure 2 ).
| Variable | Cardiac Death | p Value | |
|---|---|---|---|
| Yes (n = 32) | No (n = 74) | ||
| Seattle Heart Failure Model score | 0.50 ± 0.75 | 0.05 ± 0.65 | 0.0025 |
| Rounded Seattle Heart Failure Model score (n) | |||
| −1 | 3 | 12 | |
| 0 | 14 | 57 | |
| 1 | 12 | 16 | |
| 2 | 3 | 1 | |
| Cardiac meta-iodobenzylguanidine imaging | |||
| Heart/mediastinum ratio from early images | 1.76 ± 0.27 | 1.88 ± 0.27 | 0.0479 |
| Heart/mediastinum ratio from delayed images | 1.57 ± 0.24 | 1.79 ± 0.32 | 0.0007 |
| Washout rate | 39.4 ± 14.0 | 25.5 ± 13.5 | <0.0001 |
The results from the univariate and multivariate analyses for the identification of patients with CHF at risk of cardiac death are listed in Table 3 . A multivariate Cox analysis revealed that the WR (p = 0.0002) and SHFM score (p = 0.0091) were independent predictors of cardiac death. The Kaplan-Meier analysis ( Figure 3 ) of the subgroup with a SHFM score of ≥1 revealed that the incidence of cardiac death was significantly greater in the patients with than without an abnormal WR (67% vs 20%, respectively, p = 0.01). Furthermore, in the subgroup with a SHFM score of ≤0, the incidence of cardiac death was also significantly greater in the patients with than without an abnormal WR (37% vs 11%, respectively, p = 0.004). The relative risk of an abnormal WR for cardiac death was 3.3 (95% confidence interval 1.2 to 9.7) and 3.4 (95% confidence interval 1.2 to 9.6) in patients with a SHFM score of ≥0 and ≤1, respectively. The presence of an abnormal WR increased the risk of cardiac death by 3.3-fold for patients with a SHFM score of ≥1 and by 3.4-fold for patients with a SHFM score of ≤0.



