Several clinically available cardiac biomarkers have established their prognostic value in patients with acute coronary syndromes. However, their relative prognostic significance in stable subjects has not been prospectively validated, either individually or in combination. The aim of this study was to evaluate the extent to which B-type natriuretic peptide, myeloperoxidase, and high-sensitivity C-reactive protein alone or together could be prognostic biomarkers in 3,635 consecutive stable patients without acute coronary syndrome who underwent elective diagnostic coronary angiography. After adjusting for traditional risk factors and renal function, each of the markers monitored was a significant predictor of incident major adverse cardiovascular events (death, nonfatal myocardial infarction, and stroke) over 3 years. A cardiac biomarker score based on the sum total of “positive” biomarkers provided independent prediction of future risk for incident major adverse cardiovascular events at 3 years (hazard ratio [HR] 7.61, 95% confidence interval [CI] 4.98 to 11.65, p <0.001), even after adjusted for traditional risk factors (HR 6.11, 95% CI 3.98 to 9.38, p <0.001). A positive cardiac biomarker score remained a strong and independent predictor of 3-year risk for major adverse cardiovascular events among those with normal glycemic control (HR 4.24, 95% CI 1.96 to 9.18, p <0.001), those with prediabetes (HR 7.62, 95% CI 3.87 to 15.01, p <0.001), and those with diabetes (HR 5.61, 95% CI 2.55 to 12.33, p <0.001), as well as within subjects without significant angiographic evidence of coronary artery disease (HR 10.82, 95% CI 3.82 to 30.6, p <0.001). In conclusion, an integrated assessment of cardiac biomarkers may provide independent prognostic value for long-term adverse clinical events in stable cardiac patients.
Increasingly, cardiac biomarkers have provided important information in predicting short-term and long-term risk profiles in patients with acute coronary syndromes, particularly when used in combination. Several clinically available cardiac biomarkers, including B-type natriuretic peptide (BNP), myeloperoxidase (MPO), and high-sensitivity C-reactive protein (hsCRP), provide incremental prognostic value in patients with acute coronary syndromes, alone or in combination. Their ability to predict cardiovascular risk has been postulated, as they reflect underlying biomarkers of myocardial dysfunction, plaque vulnerability, and systemic inflammation, respectively. However, the clinical utility of such biomarkers simultaneously in a stable, nonacute patient cohort is less well established. We hypothesized that simultaneous assessment of these clinically available cardiac biomarkers to produce a risk score (composed of the sums of “positive” biomarkers on the basis of established cutoff values) would provide incremental prognostic insight into predicting future adverse cardiovascular outcomes. As there is an evolving understanding of patients with diabetes and prediabetes being at heightened cardiovascular risk, we further analyzed the prognostic utility of these cardiac biomarkers across the spectrum of glycemic control.
Methods
We prospectively evaluated 3,635 consecutively consenting subjects who underwent elective cardiac catheterization recruited from 2001 to 2006 without evidence of myocardial infarction (cardiac troponin I <0.03 ng/ml). All participants gave written informed consent, and the institutional review board of the Cleveland Clinic approved the study protocol. The Framingham risk score was calculated for each subject on the basis of Adult Treatment Panel III guidelines. An estimate of creatinine clearance was calculated using the Cockcroft-Gault equation. Coronary artery disease was defined as any clinical history of myocardial infarction, percutaneous coronary intervention, coronary artery bypass surgery, or angiographic evidence of coronary artery disease (≥50% stenosis) in ≥1 major coronary artery. Glycemic status and clinical definition of diabetes mellitus, “prediabetes,” and nondiabetes were defined by the latest practice guidelines on the basis of fasting glucose and glycosylated hemoglobin (HbA 1c ) levels (fasting glucose <100 mg/dl and HbA 1c <5.7% for normal subjects; fasting glucose ≥126 mg/dl or HbA 1c ≥6.5% or currently taking glucose-lowering medications for diabetes mellitus; neither normal nor diabetes mellitus for prediabetes). Adjudicated outcomes were ascertained over the ensuing 3 years for all subjects after enrollment. The prospective determination of clinical outcomes was made by the research personnel contacting participants independent of study investigators, with prespecified criteria and confirmation by review of documentation independent of the investigators. Major adverse cardiovascular events (MACEs) were defined as death, nonfatal myocardial infarction, or nonfatal cerebrovascular accident after enrollment. Blood samples were collected before the administration of heparin, placed on ice, aliquoted, and frozen at −80°F within 2 hours of collection. All laboratory assays, including hsCRP, BNP, MPO, apolipoprotein A1, apolipoprotein B100, and creatinine, were performed using the Abbott ARCHITECT ci8200 platform (Abbott Diagnostics Inc., Abbott Park, Illinois). The intra- and interassay coefficients were 4% and 2.4% for hsCRP, 2.6% and 3.5% for BNP, and 6.2% and 4.1% for MPO, respectively.
Student’s t tests or Wilcoxon’s rank-sum tests for continuous variables and chi-square tests for categorical variables were used to examine the differences between the groups. A cardiac biomarker score (CBS) was given to each group on the basis of whether it had a positive value for each respective biomarker. We used cutoffs for each of the 3 biomarkers (BNP >100 pg/ml, hsCRP >2.0 ng/L, and MPO >322 pmol/L) on the basis of previous cutoffs used for the respective markers, as reported in previous studies. Each of the groups was categorized as 0, 1, 2, or 3 as a measure of how many biomarkers were deemed positive, which was defined as the CBS. Kaplan-Meier analysis with Cox proportional-hazards regression was used for time-to-event analysis to determine hazard ratios (HRs) and 95% confidence intervals (95% CIs) for MACEs. Unadjusted trends for all-cause mortality rates as well as rates of nonfatal myocardial infarction or stroke with increasing quartiles of MPO, hsCRP, and BNP were evaluated using the Cochran-Armitage test using a time-to-event approach. Adjustments were made for individual traditional cardiac risk factors (including age, gender, low-density and high-density lipoprotein cholesterol, systolic blood pressure, former or current cigarette smoking, diabetes mellitus, apolipoprotein B100/apolipoprotein A1 ratio, history of myocardial infarction, and creatinine clearance) to predict incident 3-year risk for MACEs. Net reclassification analysis was performed with the 2 Cox models adjusted for traditional risk factors. Cutoff values for net reclassification index estimation used a ratio of 6:3:1 for low-, medium-, and high-risk categories. All analyses were performed using R version 8.02 (R Foundation for Statistical Computing, Vienna, Austria), and p values <0.05 were considered statistically significant.
Results
Table 1 describes the baseline characteristics of our study population and is stratified according to glycemic status. The median levels of hsCRP, BNP, and MPO were 2.00 pg/ml (interquartile range 0.91 to 4.47), 83 pg/ml (interquartile range 34 to 200), and 103 pmol/L (interquartile range 70 to 195), respectively. All 3 biomarkers were notably elevated in patients with diabetes compared to those with prediabetes or nondiabetes.
| Variable | Whole Cohort (n = 3,635) | Diabetes Mellitus (n = 1,014) | Prediabetes (n = 1,529) | Nondiabetes (n = 1,092) | p Value |
|---|---|---|---|---|---|
| Age (yrs) | 63 ± 11 | 64 ± 10 | 63 ± 11 | 61 ± 12 | <0.001 |
| Men | 65% | 61% | 70% | 64% | <0.001 |
| Hypertension | 71% | 78% | 70% | 62% | <0.001 |
| History of myocardial infarction | 33% | 35% | 32% | 32% | <0.150 |
| Systolic blood pressure (mm Hg) | 133 (120–146) | 134 (120–149) | 132 (120–145) | 132 (119–147) | <0.015 |
| Low-density lipoprotein cholesterol (mg/dl) | 95 (78–116) | 95 (77–115) | 97 (80–118) | 94 (76–116) | <0.012 |
| High-density lipoprotein cholesterol (mg/dl) | 34 (28–41) | 32 (27–39) | 34 (28–42) | 34 (29–42) | <0.001 |
| Creatinine clearance (ml/min/1.73 m 2 ) | 100 (76–126) | 99 (74–128) | 100 (77–126) | 100 (79–126) | <0.512 |
| Cigarette smoking | 65% | 64% | 68% | 62% | <0.002 |
| Aspirin | 73% | 73% | 74% | 71% | <0.357 |
| β blockers | 61% | 65% | 62% | 56% | <0.001 |
| Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers | 50% | 60% | 47% | 41% | <0.001 |
| Statins | 59% | 63% | 59% | 54% | <0.001 |
| hsCRP (mg/L) | 2.00 (0.91–4.47) | 2.56 (1.13–5.93) | 1.89 (0.86–3.95) | 1.67 (0.83–4.00) | <0.001 |
| BNP (pg/ml) | 83 (34–200) | 93 (40–240) | 78 (32–177) | 83 (32–198) | <0.001 |
| MPO (pmol/L) | 103 (70–195) | 105 (74–186) | 104 (69–201) | 100 (68–194) | <0.199 |
Table 2 represents the prognostic value of individual cardiac biomarkers in our study cohort. All 3 cardiac biomarkers provided incremental risk prediction in our study cohort. After adjusting for traditional risk factors, including Framingham risk factors, log-transformed BNP, hsCRP, and MPO each remained independent predictors of incident MACEs at 3-year follow-up.
| Variable | Univariate Model | Multivariate Model ∗ | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| BNP >100 pg/ml | 2.76 (2.25–3.39) | <0.001 | 2.10 (1.65–2.68) | <0.001 |
| hsCRP >2 ng/L | 2.10 (1.71–2.58) | <0.001 | 1.82 (1.46–2.28) | <0.001 |
| MPO >322 pmol/L | 1.43 (1.12–1.82) | <0.004 | 1.32 (1.02–1.71) | <0.036 |
∗ Adjusted for age, gender, low-density and high-density lipoprotein cholesterol, systolic blood pressure, former or current cigarette smoking, diabetes mellitus, history of myocardial infarction, and creatinine clearance.
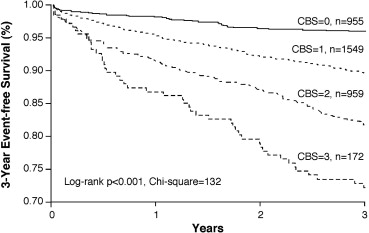
By summing the number of positive cardiac biomarkers, we developed a CBS that integrated the risk profile of our study cohort. As illustrated in Figure 1 , the CBS provided incremental prognostic value, as displayed by Kaplan-Meier analysis. As listed in Table 3 , the CBS, based on the sum total of positive biomarkers, provided independent prediction of future risk for incident MACEs at 3 years (HR 7.61, 95% CI 4.98 to 11.65, p <0.001), even after adjustment for traditional risk factors (HR 6.11, 95% CI 3.98 to 9.38, p <0.001), in addition to apolipoprotein B100/apolipoprotein A1 ratio (HR 6.11, 95% CI 3.98 to 9.38, p <0.001) ( Table 3 ). The ability of the CBS to provide incremental risk stratification can be seen in subgroups of patients with primary and secondary prevention, as well as those with maximal coronary artery stenoses <50% and ≥50% ( Figure 2 ). A higher CBS predicted future risk for MACEs at 3 years regardless of age, gender, body mass index, diabetes mellitus, hypertension, renal insufficiency, or previous myocardial infarction (p <0.01 for all). Use of the CBS on top of traditional risk factors was also shown to reclassify subjects (net reclassification index 12.86%, p <0.001; integrated discrimination improvement 12.0%, p <0.001; C-statistic 66.9% vs 71.1%, p <0.001).
| Variable | CBS | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Whole cohort (n = 3,635) | ||||
| Unadjusted HR | 1 | 2.59 (1.82–3.68) ∗ | 4.72 (3.33–6.69) ∗ | 7.61 (4.98–11.65) ∗ |
| Adjusted HR, model 1 (95% CI) | 1 | 2.27 (1.59–3.23) ∗ | 3.67 (2.58–5.24) ∗ | 6.11 (3.98–9.38) ∗ |
| Adjusted HR, model 2 (95% CI) | 1 | 2.27 (1.59–3.23) ∗ | 3.67 (2.58–5.24) ∗ | 6.11 (3.98–9.38) ∗ |
| MACEs | 39/955 | 159/1,549 | 173/959 | 47/172 |
| Normal (n = 1,014) | ||||
| Unadjusted HR | 1 | 1.78 (0.94–3.37) | 3.23 (1.69–6.16) ∗ | 6.23 (2.9–13.37) ∗ |
| Adjusted HR, model 1 (95% CI) | 1 | 1.43 (0.74–2.75) | 2.37 (1.21–4.64) ∗ | 4.73 (2.18–10.25) ∗ |
| Adjusted HR, model 2 (95% CI) | 1 | 1.36 (0.7–2.61) | 2.23 (1.14–4.39) ∗ | 4.24 (1.96–9.18) ∗ |
| MACEs | 13/290 | 35/445 | 32/230 | 13/49 |
| Prediabetes (n = 1,529) | ||||
| Unadjusted HR | 1 | 2.74 (1.56–4.84) ∗ | 4.75 (2.7–8.38) ∗ | 10.27 (5.24–20.13) ∗ |
| Adjusted HR, model 1 (95% CI) | 1 | 2.4 (1.36–4.23) ∗ | 3.67 (2.06–6.54) ∗ | 7.87 (3.99–15.55) ∗ |
| Adjusted HR, model 2 (95% CI) | 1 | 2.37 (1.35–4.19) ∗ | 3.58 (2–6.41) ∗ | 7.62 (3.87–15.01) ∗ |
| MACEs | 15/427 | 61/654 | 61/379 | 20/69 |
| Diabetes mellitus (n = 1,092) | ||||
| Unadjusted HR | 1 | 3.17 (1.67–6) ∗ | 5.59 (2.98–10.49) ∗ | 6.16 (2.8–13.52) ∗ |
| Adjusted HR, model 1 (95% CI) | 1 | 3.06 (1.61–5.78) ∗ | 4.79 (2.55–9) ∗ | 5.59 (2.54–12.31) ∗ |
| Adjusted HR, model 2 (95% CI) | 1 | 3.05 (1.61–5.77) ∗ | 4.8 (2.55–9.01) ∗ | 5.61 (2.55–12.33) ∗ |
| MACEs | 11/238 | 63/450 | 80/350 | 14/54 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


