Substantial evidence points to a protective role of adiponectin against atherosclerosis and cardiovascular (CV) disease. However, in the setting of an acute myocardial infarction (AMI), the role of adiponectin has not previously been studied. Consequently, the aim of this study was to investigate the prognostic role of adiponectin after AMI in a large population of patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. A total of 735 consecutive patients with ST-segment elevation myocardial infarction admitted to a single high-volume invasive heart center and treated with primary percutaneous coronary intervention from September 2006 to December 2008 were included. Blood samples were drawn immediately before the invasive procedure. Plasma adiponectin was measured using a validated immunoassay. End points were all-cause mortality, CV mortality, and admission for new AMI or heart failure. The median follow-up time was 27 months (interquartile range 22 to 33). Patients with high adiponectin (quartile 4) had increased mortality compared to patients with low adiponectin (quartiles 1 to 3) (log-rank p <0.001). After adjustment for conventional risk factors (age, gender, smoking, hypertension, hypercholesterolemia, diabetes, body mass index, C-reactive protein, peak troponin I, creatinine, estimated glomerular filtration rate, previous AMI, multivessel disease, complex lesions, left anterior descending coronary artery lesion, and symptom-to-balloon time) by Cox regression analysis, high adiponectin remained an independent predictor of all-cause mortality (hazard ratio 2.1, 95% confidence interval 1.3 to 3.2, p = 0.001) and CV mortality (hazard ratio 2.6, 95% confidence interval 1.5 to 4.5, p = 0.001). In conclusion, increased plasma adiponectin independently predicts all-cause and CV mortality in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention.
Epidemiologic studies of healthy subjects have shown an association between high adiponectin and decreased risk for cardiovascular (CV) disease (CVD). However, in the presence of existing CVD, high levels of adiponectin are associated with increased mortality, although not all studies have found this association. Considering these divergent and paradoxical data, the aim of the present study was to further elucidate the role of adiponectin and mortality in high-risk patients with CVD. Accordingly, we conducted a prospective cohort study of patients with ST-segment elevation myocardial infarction (STEMI), investigating the relation between adiponectin and all-cause mortality and CV mortality. Furthermore, we aimed to examine the association of adiponectin and admission for new myocardial infarction or heart failure.
Methods
We prospectively enrolled 735 consecutive patients with STEMI treated with primary percutaneous coronary intervention (PCI) from September 2006 through December 2008 at Gentofte University Hospital (Copenhagen, Denmark). Inclusion criteria were presence of chest pain for >30 minutes and <12 hours, a significant increase in troponin I (>0.5 μg/L), and persistent ST-segment elevation ≥2 mm in ≥2 contiguous precordial electrocardiographic leads or ≥1 mm in ≥2 contiguous limb electrocardiographic leads. A limited number of patients (n = 10) were included for newly developed left bundle branch block. A description of the PCI setting and procedure at Gentofte University Hospital has previously been published. In brief, it is a high-volume PCI center performing >1,500 PCI procedures a year (including approximately 500 primary PCI procedures) with on-site cardiac surgery. Subsequent medical treatment included aspirin 75 mg/day, clopidogrel 75 mg/day (for 12 months), lipid-lowering drugs (statins), and β-receptor antagonists.
Hypertension was defined as use of blood pressure–lowering drugs at admission. Hypercholesterolemia was defined as patients receiving current treatment with cholesterol-lowering medications. Patients taking glucose-lowering drugs at admission, with fasting plasma glucose concentrations ≥7 mmol/L, or with nonfasting plasma glucose concentrations ≥11.1 mmol/L were considered to have diabetes mellitus. Body mass index was calculated as weight in kilograms divided by the square of height in meters. Multivessel disease was defined as 2- or 3-vessel disease, and complex lesions were defined as type C lesions.
Patients were followed for a median of 27 months (interquartile range 22 to 33). Follow-up information was obtained on death, cause of death, and admission for new myocardial infarction or heart failure. Information was obtained from the National Person Identification Registry and collected using hospital source data, as well as data from the highly validated Danish National Board of Health National Patient Registry. Follow-up information was obtained for 99.9% of the patients (n = 734). Echocardiography was performed at our institution in 367 patients (50%) using Vivid 7 or E9 (GE Vingmed Ultrasound AS, Horten, Norway) 1 to 2 days after the infarction. The left ventricular ejection fraction was visually estimated by expert interpreters guided by strain analyses and wall motion index, which has previously been demonstrated to be a very reliable method.
Blood samples were drawn from the femoral sheath at the beginning of the primary PCI procedure, thus avoiding contamination with contrast fluid. Serum was centrifuged within 30 minutes, and plasma was stored at −80°C. Plasma adiponectin was determined using a validated in-house time-resolved immunofluorometric assay as previously described. All samples were analyzed in duplicate, with a detection limit of 1.5 μg/L and intra- and interassay coefficients of variation of <5% and <7%, respectively. Other blood tests, including C-reactive protein, lipids, troponin I, and creatinine, were assayed using routine laboratory methods. Troponin I was measured at admission and after 6 and 12 hours. Glomerular filtration rate was estimated on the basis of serum creatinine, age, and gender using the Cockcroft-Gault formula as previously described.
Adiponectin displayed a non-Gaussian distribution, and thus the patient population was stratified into quartiles. Proportions were compared using chi-square tests, continuous Gaussian-distributed variables using Student’s t tests, and non-Gaussian-distributed variables using Mann-Whitney U tests. Associations between adiponectin and other variables were tested using univariate logistic or linear regression.
The association of adiponectin with survival was examined using Cox proportional-hazards regression analyses. The assumptions of linearity and proportional hazards in the models were ensured. First-order interactions between adiponectin and other variables (age, gender, smoking, hypertension, hypercholesterolemia, diabetes, body mass index, C-reactive protein, peak troponin I, creatinine, estimated glomerular filtration rate, previous myocardial infarction, left ventricular ejection fraction, multivessel disease, complex lesions, left anterior descending coronary artery lesion, and symptom-to-balloon time) for end points were assessed. To maintain a robust model, only 1 variable for each 10 events was allowed in the multivariate Cox analyses of each end point. Variables were chosen using backward elimination. Kaplan-Meier curves according to quartiles (quartiles 1 to 3 vs quartile 4) of adiponectin levels were constructed and compared using log-rank tests. In the statistical tests, p values ≤0.05 were considered of statistical significance. SPSS for Windows version 18.0 (SPSS, Inc., Chicago, Illinois) was used. The study was approved by the local scientific ethics committee and the Danish Data Protection Agency and complied with the second Declaration of Helsinki.
Results
During a median follow-up time of 27 months (interquartile range 22 to 33), the total mortality was 13.5%, 50 patients (7%) died from CV causes, 49 (7%) died from non-CV causes, 52 (7%) had new myocardial infarctions, and 68 (9%) were admitted to the hospital for heart failure. The median adiponectin level was 6.9 mg/L (interquartile range 5.0 to 10.2). High adiponectin was significantly associated with female gender and inversely associated with current smoking, hypercholesterolemia, serum creatinine, and impaired renal function as assessed by estimated glomerular filtration rate ( Table 1 ).
| Variable | Adiponectin | p Value | |
|---|---|---|---|
| Quartiles 1–3 | Quartile 4 | ||
| (n = 551) | (n = 184) | ||
| Age (years) | 61 ± 12 | 69 ± 12 | 0.24 |
| Men | 82% | 50% | <0.001 |
| Hypertension | 33% | 36% | 0.38 |
| Diabetes mellitus | 11% | 6% | 0.07 |
| Current smoker | 53% | 44% | 0.03 |
| Hypercholesterolemia | 22% | 11% | 0.001 |
| Previous myocardial infarction | 7% | 3% | 0.10 |
| Known heart failure | 3.3% | 2.7% | 0.71 |
| Body mass index (kg/m 2 ) | 27.5 ± 4.4 | 24.4 ± 4.1 | 0.12 |
| Peak troponin I (μg/L) | 89 (29–243) | 134 (26–256) | 0.41 |
| C-reactive protein (mg/L) | 3.0 (2.0–9.0) | 4.0 (1.0–10.0) | 0.85 |
| Serum creatinine (μmol/L) | 92 (79–208) | 89 (72–110) | 0.049 |
| Estimated glomerular filtration rate (ml/min) | 74 (61–88) | 71 (53–86) | 0.03 |
| Symptom-to-balloon time (min) | 196 (127–330) | 196 (135–315) | 0.97 |
| Left ventricular ejection fraction (%) (available in 50% [n = 367]) | 40 (30–50) | 40 (25–50) | 0.31 |
| Complex lesion | 49% | 50% | 0.82 |
| Multivessel disease | 27% | 27% | 0.95 |
| Left anterior descending coronary artery lesion | 45% | 46% | 0.68 |
| Glycoprotein IIb/IIIa inhibitor | 27% | 22% | 0.26 |
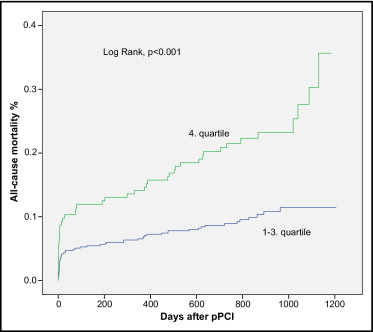
No interactions between adiponectin and other baseline variables with respect to reaching an end point were found. Log-rank tests on the basis of the Kaplan-Meier curves showed significant associations between high adiponectin and all-cause mortality (p <0.001; Figure 1 ) and CV mortality (p <0.001). This association was confirmed in univariate Cox analysis ( Table 2 ). Even after adjustment for other variables, adiponectin remained a significantly independent predictor of all-cause and CV mortality ( Table 3 ). No association between high adiponectin and admission for new myocardial infarction or heart failure was found ( Tables 2 and 3 ).
| Variable | Hazard Ratio | 95% Confidence Interval | p Value |
|---|---|---|---|
| All-cause mortality (n = 99) | 2.56 | 1.72–3.80 | <0.001 |
| Cardiovascular mortality (n = 50) | 2.85 | 1.63–4.93 | <0.001 |
| New myocardial infarction (n = 52) | 0.61 | 0.30–1.25 | 0.18 |
| Heart failure (n = 68) | 1.05 | 0.61–1.83 | 0.85 |
| Variable | Hazard Ratio | 95% Confidence Interval | p Value |
|---|---|---|---|
| All-cause mortality (n = 99) ⁎ | 2.07 | 1.34–3.20 | 0.001 |
| Cardiovascular mortality (n = 50) † | 2.57 | 1.46–4.50 | 0.001 |
| New myocardial infarction (n = 52) ‡ | 0.66 | 0.32–1.36 | 0.26 |
| Heart failure (n = 68) § | 1.03 | 0.58–1.85 | 0.92 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


