International guidelines do not specify what testing should be performed during emergency department (ED) evaluations for patients presenting with an exacerbation of previously diagnosed atrial fibrillation (AF). We hypothesized that low CHADS2 and CHA2DS2-VASc scores predict normal routine diagnostic testing in these patients. We conducted an analysis within a prospective observational cohort study at a university-affiliated hospital. We included patients with previously diagnosed AF and who presented to the ED primarily for an AF-related complaint. Logistic regression was used to analyze the association between CHADS2 and CHA2DS2-VASc scores and abnormal results for blood counts, electrolytes, cardiac markers, thyroid function, and chest x-rays. We included 216 patients in this analysis. The odds ratios (95% confidence interval) for each point increase in CHADS2 for abnormal blood counts, electrolytes, troponin I, brain natriuretic peptide, thyroid function, and chest x-ray were 1.28 (0.99 to 1.65), 1.48 (1.19 to 1.84), 1.42 (1.10 to 1.82), 1.66 (1.15 to 2.41), 0.95 (0.70 to 1.29), and 1.17 (0.94 to 1.44), respectively. The corresponding odds ratios (95% confidence interval) for each point increase in CHA2DS2-VASc were 1.17 (0.96 to 1.42), 1.27 (1.09 to 1.49), 1.30 (1.07 to 1.57), 1.57 (1.18 to 2.10), 0.98 (0.79 to 1.22), and 1.14 (0.97 to 1.33), respectively. Among ED patients with established AF who underwent evaluation for acutely symptomatic AF, nearly 3/4 of routine diagnostic tests return to normal. In conclusion, patients with CHADS2 or CHA2DS2-VASc score of 0 have the lowest likelihood of abnormal studies and may represent an easily identifiable group of patients who need fewer ED tests.
Atrial fibrillation (AF) affects 3 to 6 million patients in the United States with annual healthcare costs of $6 to $26 billion. Emergency department (ED) services trail only inpatient services and office visits as the principal driver of medical costs. A common course for patients with established AF is that the disease becomes a chronic condition with frequent exacerbations leading to ED evaluations and hospitalizations during which the same diagnostic studies are frequently repeated. International guidelines for managing AF do not provide specific recommendations for ED management and diagnostic testing. The guidelines do state that frequent assessment of a patient’s risk for thromboembolism, using the CHADS2 (Congestive heart failure (1 point), Hypertension (1 point), Age 75y (1 point), Diabetes (1 point), prior Stroke/transient ischemic attack (2 points)) and CHA2DS2-VASc (Congestive heart failure (1 point), Hypertension (1 point), Age 75y (2 points), Diabetes (1 point), prior Stroke/transient ischemic attack (2 points)-Vascular disease [prior myocardial infarction, peripheral artery disease, or aortic plaque] (1 point), Age 65–74y (1 point), Sex category [i.e., female gender] (1 point)) scores, is a main objective in AF management. Studies have shown that the CHADS2 and CHA2DS2-VASc scores are predictive of a wide array of important clinical outcomes in patients with and without AF. The CHADS2 and CHA2DS2-VASc scores may be sufficient measures of increased overall risk to identify patients who may be safely managed with limited testing. We investigated the frequency of diagnostic testing and the prevalence of abnormal results in a prospective cohort of patients with a previous history of AF who presented to the ED with primary AF-related complaints. We also evaluated the performance of the CHADS2 and CHA2DS2-VASc scores to predict which patients have abnormal results to common diagnostics tests.
Methods
We conducted a prospective observational cohort study (NCT01138644) at a single, tertiary care, university-affiliated hospital adult ED that treats 65,000 adult patients annually. This study is a secondary investigation of the Atrial Fibrillation/Flutter Outcome Risk Determination study, the details of which have been previously reported. Briefly, the research team identified patients presenting with signs (e.g., tachycardia, dyspnea) and symptoms (e.g., palpitations, chest pain, shortness of breath, weakness, lightheadedness, presyncope, or syncope) consistent with symptomatic AF or atrial flutter. We enrolled the first subject on June 8, 2010 and completed enrollment on February 28, 2013. The diagnosis of AF was confirmed by an independent cardiologist’s review of the electrocardiogram performed on the day of the patient’s ED evaluation. We classified each patient’s AF type as newly diagnosed or an established diagnosis (which included paroxysmal, persistent, or permanent AF) based on the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. The decision to perform diagnostic testing in the ED was at the discretion of the treating clinician.
We focused our analysis on patients with an established diagnosis of AF as these patients previously underwent the recommended battery of testing aimed to exclude an underlying cause (e.g., structural heart disease, thyroid disease) of their AF. Patients with newly diagnosed AF, however, may have little previous diagnostic testing data available and the treating clinician may want additional laboratory and x-ray information before making disposition and treatment decisions. We included only patients whose ED evaluations were primarily for AF. To determine whether the ED evaluation was primarily for an AF, a board-certified emergency medicine physician investigator reviewed the ED records of all patients who consented for the study. The principal investigator used a standardized protocol to determine whether uncontrolled AF was the primary reason for the ED visit. A board-certified electrophysiologist investigator provided a second review of a randomly selected 31% subset of the enrollments. We measured the absolute agreement and Cohen’s kappa statistic between the 2 reviewers’ designation for the primary cause of ED visits. We calculated 95% confidence intervals (CIs) for the kappa using 1,000 bootstrap resamples.
We collected data on all diagnostic testing performed in the ED during each subject’s evaluation, including electrocardiograms, laboratory investigations, and x-ray studies. We focused this investigation on the following commonly ordered ED diagnostic studies: complete blood counts (CBCs), basic metabolic panel, troponin I, brain natriuretic peptide (BNP), thyroid function tests, and the chest x-ray. We made an a priori determination that the following diagnostic tests should be considered standard clinical practice and did not include them in the analysis: bedside glucose testing in patients with known diabetes, creatinine in patients with chronic kidney disease and BNP testing in patients with established heart failure. We used normal range references published on the National Library of Medicine’s Medline Plus and National Endocrine and Metabolic Diseases Information Service websites to categorize each patient’s laboratory investigations as within the normal or abnormal range (i.e., outside the normal range of values). A detailed description of what constituted a normal and abnormal test is listed in the Appendix Table 1 .
We calculated CHADS2 and CHA2DS2-VASc scores for each patient using medical history data obtained through both patient interview and medical record review at the time of the ED evaluation. We investigated both the CHADS2 and CHA2DS2-VASc scores. The CHADS2 score remains the preferred predictor for thromboembolic risk in the United States. The most recent 2012 European Society of Cardiology guidelines recommended that the CHA2DS2-VASc score replace the CHADS2 as studies have shown it to be superior in identifying truly low-risk patients.
This investigation’s primary outcome variable was the proportion of patients with abnormal results for these routine diagnostic tests: CBC, basic metabolic panel, troponin I, BNP, thyroid function tests, and chest x-ray. CHADS2 and CHA2DS2-VASc scores were the primary exposure variables. CHADS2 score categories included scores of 0, 1, 2, 3, and 4 to 6. Scores 4 to 6 were shrunken into one category because of small sample sizes with these high scores and similar clinical significance of scores ≥4. Similarly, CHA2DS2-VASc score categories included scores of 0, 1, 2, 3, 4, 5, and 6 to 9. We calculated the proportion of patients in each CHADS2 and CHA2DS2-VASc category with abnormal results for each of the routine diagnostic test. We constructed a series of simple logistic regression models. In each model, either CHADS2 or CHA2DS2-VASc score category was the independent variable. Dependent variables included dichotomized (normal or abnormal) diagnostic test results for CBC, basic metabolic panel, troponin I, BNP, thyroid function tests, and chest x-ray, with each test considered in a separate model. Therefore, 12 separate simple logistic regression models were constructed with 6 having CHADS2 as the independent variable and 6 having CHA2DS2-VASc as the independent variable. Model output included the odds ratio with 95% CI for the association between CHADS2 or CHA2DS2-VASc score category and an abnormal diagnostic test.
In secondary analyses, we also dichotomized CHADS2 and CHA2DS2-VASc scores as 0 or ≥1. This dichotomization was based on previous literature that classified patients with CHADS2 and CHA2DS2-VASc scores of 0 as lowest risk for thromboembolism. We then calculated the proportion of patients with these dichotomized CHADS2 and CHA2DS2-VASc categories with abnormal diagnostic tests. CIs for these proportions were calculated by Wilson’s method for calculating binomial proportion interval estimates. Statistical analyses were performed with Stata 12 (College Station, Texas) and IBM SPSS Version 21 (Armonk, New York). Our medical center’s institutional review board approved this study and patients provided written informed consent.
Results
Between June 2010 and February 2013, we prospectively enrolled 519 patients in the Atrial Fibrillation/Flutter Outcome Risk Determination study including 334 patients whose ED visits were for primary AF. Of them, 216 (64.7%) patients having AF as both a previously established diagnosis and the primary reason for ED presentation constituted the study cohort for this analysis. The principal investigator adjudicated whether AF was the primary indication for the ED visit in all patients. An electrophysiologist independently reviewed 163 (31.4%) of the records. Of those 163 records, concordance was found in 139, corresponding to a kappa of 0.79 (95% CI 0.70 to 0.88).
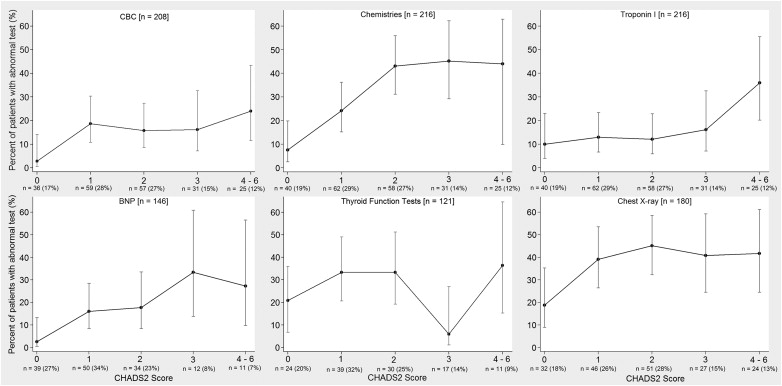
Table 1 lists the baseline characteristics for the 216 patients included in this study. Table 2 lists the frequency of diagnostic studies performed in the ED and the percentages of abnormal results. The odds ratios for CHADS2 and CHA2DS2-VASc scores predicting an abnormal result for each routine diagnostic study are listed in Table 3 . Figures 1 and 2 show the proportion of patients who had abnormal diagnostic tests in the ED stratified by CHADS2 and CHA2DS2-VASc scores. Table 4 lists that for those with CHADS2 or CHA2DS2-VASc score of 0, CBC, basic metabolic panel, and BNP each were abnormal in <10% of patients. Additional details on the patient’s baseline characteristics and ED evaluations are listed in the Appendix Tables 2 and 3 .
| Variable | Study Population (n = 216) |
|---|---|
| Age (yrs) | 65.5 (57, 75) |
| Women | 84 (39) |
| White | 192 (89) |
| Black | 22 (10) |
| Chief complaint in triage | |
| Palpitations | 89 (41) |
| Chest pain | 35 (16) |
| Dyspnea | 25 (12) |
| Tachycardia | 28 (13) |
| Syncope | 7 (3) |
| Blood pressure, systolic (mm Hg) | 132 (117, 147) |
| Blood pressure, diastolic (mm Hg) | 82 (72, 91) |
| Heart rate (beats/min) | 120 (95, 137) |
| Duration of symptoms before ED presentation | |
| <48 hours | 147 (50) |
| >48 hours | 49 (23) |
| Unknown | 20 (9) |
| CHADS2 score | 2 (1, 3) |
| CHA2DS2-VASc score | 3 (1, 4) |
| Type of atrial fibrillation | |
| Paroxysmal | 153 (71) |
| Persistent | 38 (18) |
| Permanent | 25 (11) |
| Medical history | |
| Heart failure | 62 (29) |
| Myocardial infarction | 45 (21) |
| Coronary artery disease | 68 (32) |
| Hypertension | 148 (69) |
| Diabetes mellitus | 48 (22) |
| Peripheral vascular disease | 18 (8) |
| Cerebrovascular accident | 26 (12) |
| Transient ischemic attack | 17 (8) |
| Variable | Patients With Test Completed (n = 216) | Patients With Abnormal Result Among Patients With Test Completed (n = 216) |
|---|---|---|
| CBC | 208 (96) | 32 (15) |
| Basic metabolic panel | 216 (100) | 68 (31) |
| Troponin I | 216 (100) | 33 (15) |
| BNP | 146 (68) | 22 (15) |
| Thyroid function tests | 121 (56) | 33 (27) |
| Chest x-ray | 180 (83) | 68 (38) |
| Variable | Abnormal CBC (n = 208) | Abnormal Basic Metabolic Panel (n = 216) | Abnormal Troponin I (n = 216) | Abnormal BNP ∗ (n = 146) | Abnormal Thyroid Function Testing (n = 120) | Abnormal Chest X-Ray (n = 179) |
|---|---|---|---|---|---|---|
| CHADS2 | 1.28 (0.99–1.65) | 1.48 (1.19–1.84) | 1.42 (1.10–1.82) | 1.66 (1.15–2.41) | 0.95 (0.70–1.29) | 1.17 (0.94–1.44) |
| CHA2DS2-VASc | 1.17 (0.96–1.42) | 1.27 (1.09–1.49) | 1.30 (1.07–1.57) | 1.57 (1.18–2.10) | 0.98 (0.79–1.22) | 1.14 (0.97–1.33) |
∗ Patients with known history of heart failure excluded because of likelihood of chronically elevated BNP levels.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


