The ablation of ventricular tachycardia (VT) can be achieved using anatomically guided approaches using differentiated mapping and ablation techniques. The aim of this study was to evaluate the efficacy of limited linear ablation in the VT exit region identified during sinus rhythm mapping alone. One hundred fifteen consecutive patients presenting for ablation of post–myocardial infarction VT were included. After induction of the target VT during invasive electrophysiology, left ventricular substrate mapping during sinus rhythm to identify scar and border zone on the basis of endocardial bipolar voltage was performed. The exit site of the target VT was regionalized by a simplified vector pace mapping approach and targeted using limited linear ablation within the scar border zone. Seventy-seven percent of all inducible VT was successfully ablated. In 71 patients (62%), no sustained VT was inducible at the end of ablation procedure (complete success). During a median follow-up period of 16 ± 10 months, 89 patients (77%) had no documented sustained ventricular arrhythmia. Seven patients (2%) had recurrences of the initially ablated VT, and 16 (14%) had new-onset VT. Patients with complete success had a significantly lower number of ventricular arrhythmia reoccurrences than patients with incomplete ablation success (11% vs 37%, p = 0.002). In conclusion, postinfarct VT was effectively ablated in 97% of patients without mapping during ongoing VT using a simplified regional linear ablation approach targeting the scar border zone. Freedom from any ventricular arrhythmia was achieved in 77% of patients during midterm follow-up.
The annual incidence of ventricular tachycardia (VT) in patients after myocardial infarction may be as high as 2% per year. Endocardial ablation can effectively suppress recurrent tolerable postinfarct VT and is documented to reduce implantable cardioverter-defibrillator (ICD) therapy. Ablation strategies aiming to detect slowly conducting channels (isthmus) critical for the VT reentry circuit are often hampered by hemodynamic or electrical instability of the target arrhythmia. Anatomically based ablation approaches characterizing scar regions and normal myocardium during sinus rhythm (SR) mapping have evolved, minimizing the need for mapping during ongoing VT. Although VT ablation is increasingly used at experienced centers, no standardized approach and data on larger patient cohorts are available thus far. The aim of the present study was to demonstrate that postinfarct reentrant VT may be successfully ablated using a standardized protocol without mapping during ongoing VT.
Methods
Consecutive patients with recurrent monomorphic postinfarct VT and indications for invasive ablation therapy were included. Patients (n = 26) were excluded if the origin of VT was not within the left ventricle, was focal, or was documented to be epicardial ; if no VT could be induced during electrophysiology matching the index VT cycle length ; or if VT was ongoing during the invasive study. All patients gave informed consent. The study was approved by the local institutional review committee.
Electrophysiology was performed by programmed ventricular stimulation using up to 3 extrastimuli to document the 12-lead electrocardiogram of inducible monomorphic VT. If this protocol did not induce monomorphic VT, left ventricular programmed stimulation (n = 3) or burst stimulation was performed. If monomorphic VT was induced, the arrhythmia was terminated, and left ventricular substrate mapping and ablation were started. If during the termination of VT (burst pace termination) or pace mapping different sustained monomorphic VT was documented, this VT was targeted in a second step. After ablation of targeted VT, inducibility was retested by electrophysiology (using the same protocol), and if another or the same episode of VT was induced, further ablation was initiated. Different episodes of VT were assumed if electrocardiographic morphology differed. The invasive therapy was stopped if no further VT was inducible or if nontolerated monomorphic or polymorphic VT or ventricular fibrillation needing external defibrillation was induced ≥2 times (these patients were categorized as having “incomplete ablation success”) on the basis of the discretion of the investigator.
“Index VT” was defined by either comparing 12-lead electrocardiographic morphology to electrocardiography during clinically documented VT (complete correspondence of electrocardiographic morphology) or by comparison of cycle lengths as documented by ICD Holter monitoring (difference of cycle lengths <20 ms). If 2 episodes of VT with correspondence of cycle length to ICD Holter results were documented, the first induced VT episode was considered the index episode of VT. The index episode of VT was the predominantly targeted episode. In 8 patients, fast VT (cycle length ≤280 ms) was targeted as index VT.
Left ventricular endocardial mapping was performed (with heparin intravenously administered to achieve an activated clotting time >250 seconds) using Carto technology (Biosense Webster, Diamond Bar, California) during SR or ventricular paced rhythm in patients with atrioventricular conduction disturbances (called SR mapping for simplification), as previously described. The mapping procedure was directed toward the approximated exit region of the VT (as indicated by 12-lead VT electrocardiographic morphology), not to delineate complete left ventricular volume. Left ventricular endocardial bipolar electrographic amplitudes were used to regionally characterize left ventricular damage into normal myocardium (bipolar electrographic amplitude >1.5 mV), scar (<0.5 mV), and intermediately damaged myocardium (scar border zone; 0.5 to 1.5 mV).
The 12-lead electrocardiogram of the target VT was assessed to define the approximated region of the left ventricular substrate serving as an exit site of reentrant VT. In the region of interest, the border of infarction scar was identified, and stepwise vector pace mapping along the scar border zone was performed. Vector pace mapping was performed at a cycle length equal to the target VT up to a minimum of 300 ms (for VT with cycle length <300 ms). Correspondence between 12-lead VT electrocardiography and vector pace mapping was determined for each pacing site, and the regions with best pace maps were determined. Visual estimates of the QRS vector direction during pacing and VT were determined and classified as positive, negative, or isoelectric. Vector pace mapping correspondence (match) was classified only by accordance of QRS vector in each lead compared with the target VT electrocardiogram. No classification of QRS waveforms was performed. The mapping point with the best pace map was identified by the largest number of corresponding QRS vectors (within each of the 12 electrocardiographic leads). Stepwise vector pace mapping (smallest accessible step size) along the scar border away from the best pace map was acquired until a discrepancy (change in QRS vector direction) of QRS vectors occurred in ≥1 lead ( Figure 1 ).
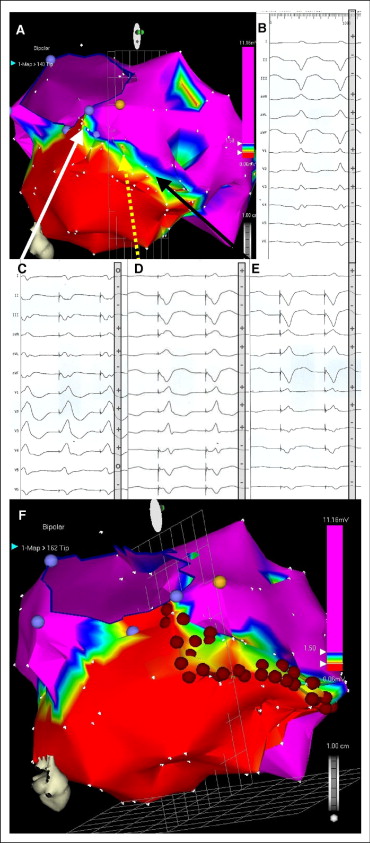
Catheter ablation was performed using a 3.5-mm-tip irrigated (20 ml/min) catheter (NaviStar ThermoCool; Biosense Webster) (48°C, 40 W) in temperature-controlled mode. Linear ablation along the scar border was directed to regions defined as intermediately damaged myocardium (bipolar voltage 0.5 to 1.5 mV) including the site of the best pace map extending toward the 2 (neighboring) adjacent pace map sites with less pace map correspondence (or extending toward anatomic landmarks such as the mitral valve annulus if within 0.5 cm of the end point of the ablation line; see Figure 1 ). Ablation was limited to areas with documented myocardial damage as close to the scar core (<0.5 mV) as accessible, preventing ablation within normal myocardium ( Figure 1 ). Local ablation was delivered for ≤90 seconds or until local bipolar electrographic amplitude decreased by 50% or <0.5 mV.
If after the completion of regional scar encircling the same episode of VT (judged by electrocardiographic morphology) was inducible, ablation was directed toward gaps (local bipolar electrogram >0.5 mV) in the ablation line and extended further beyond the ends of the ablation line (≤1 cm) to dissect broader VT exit areas. If a different VT morphology was induced, regionalization and ablation were performed as indicated.
All patients without ICDs implanted at time of ablation received devices <48 hours after the ablation procedures. All patients were monitored until ICDs were implanted. No increase in antiarrhythmic medication was allowed within the first 6 months after ablation.
Follow-up was prospectively performed every 3 months after ablation, including clinical evaluation, ICD Holter interrogation, 12-lead electrocardiography, and 24-hour Holter electrocardiography to identify electrocardiographic morphology in patients with episodes of ventricular arrhythmia. Any episode of ventricular arrhythmia (>30 seconds) and/or implementing ICD therapy was documented if identified by the implanted ICD. ICDs were individually programmed after ablation to detect the slowest induced episode of VT (cycle length + 40 ms). Episodes were judged to be ventricular arrhythmia independently by 3 experienced physicians (TD, TL, BL).
Recurrence of the index episode of VT was defined as documented monomorphic VT (>30 seconds or treated by an ICD) with a cycle length equal (±30 ms) or longer (>30 ms) (under unchanged antiarrhythmic medication). Episodes of VT with cycle lengths shorter (>30 ms) than the index episode at ablation were defined as new-onset VT.
All data are expressed as mean ± SD (range) for parametric variables and as median ± SD (range) for nonparametric variables. Statistical analysis was performed using SPSS version 15.0 (SPSS, Inc., Chicago, Illinois). Student’s t test was used to compare intergroup differences for parametric data, and Wilcoxon’s test was used for nonparametric data. The Kaplan-Meier method was used to determine freedom from any sustained ventricular arrhythmia and the log-rank test to compare intergroup differences. A p value <0.05 was considered statistically significant.
Results
From June 2004 to August 2007, a total of 115 consecutive patients with VT late after myocardial infarction (mean 7 ± 5 years) were included at the University Heart Centre Bochum (BG University Hospital Bergmannsheil) ( Table 1 ).
| Variable | Mean ± SD | Range |
|---|---|---|
| Ejection fraction (%) | 34 ± 13 | 5–70 |
| Age (years) | 66 ± 9 | 28–84 |
| Clinical VT cycle length (ms) | 382 ± 110 | 250–680 |
| Number of VT episodes in 3 months | 21.5 ± 40 | 4–220 |
| Number of episodes of inducible VT | 2.6 ± 1.6 | 1–7 |
| Procedure duration (minutes) | 162 ± 45 | 60–264 |
| Radiation exposure (minutes) | 21.2 ± 14.5 | 7–83 |
| Ablation duration (seconds) | 863 ± 425 | 159–2,141 |
| Length of ablation line (mm)/target, VT | 37 ± 7 | 16–47 |
| Median follow-up (months) | 16 ± 10 | 12–50 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


