Impact on mortality is the hardest to determine but unquestionably the most important outcome measure for clinical trials in general and for vascular occlusive studies in particular. Importantly, oral antiplatelet agents belong to the elite class of pharmaceuticals known to significantly reduce mortality in randomized outcome-driven clinical studies. Historically, only 2 placebo-controlled clinical trials, 1 with aspirin and another with clopidogrel, were associated with rare absolute mortality reduction benefits in patients after acute vascular thrombotic events. Recently, a remarkable and somewhat surprising achievement has been reported with ticagrelor in the Platelet Inhibition and Clinical Outcome (PLATO) trial, when 107 more lives were saved with ticagrelor then after conventional clopidogrel (399 vs 506), representing a highly significant absolute mortality reduction (hazard ratio 0.78, 95% confidence interval 0.69 to 0.89, p <0.001). However, being a first-in-class cyclopentyl-triazolo-pyrimidine, ticagrelor is not a pure antiplatelet agent, and it may exhibit numerous additional properties beyond platelet inhibition via the modulation of plasma adenosine levels and the upregulation of adenosine receptors. In contrast to aspirin in the Second International Study of Infarct Survival (ISIS-2), clopidogrel in the Clopidogrel and Metoprolol in Myocardial Infarction Trial (COMMIT), and ticagrelor in PLATO, prasugrel failed to show a mortality benefit over clopidogrel in the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition (TRITON), although the 2 trials did not have the required statistical power for mortality.
TRITON–Thrombolysis In Myocardial Infarction (TIMI) 38 was a phase 3, randomized, double-blind, parallel-group, multinational clinical study. With background therapy with aspirin in all patients, the trial assessed head to head the efficacy and safety of a novel thienopyridine, prasugrel, compared to standard care with clopidogrel. Patients (n = 13,608) with moderate- to high-risk acute coronary syndromes and predefined coronary anatomy who underwent percutaneous coronary intervention were randomized to prasugrel or clopidogrel and followed for 6 to 15 months. The primary end point was the rate of cardiovascular death, nonfatal myocardial infarction, or stroke and was reached in 12.1% of patients treated with clopidogrel and 9.9% of patients randomized to prasugrel, suggesting an impressive vascular outcome benefit of prasugrel over clopidogrel. However, this overoptimistic interpretation of the trial results was clouded by a United States Food and Drug Administration (FDA) secondary review, which revealed several shortcomings with TRITON’s design and data interpretation, including delayed event adjudication, the front-loaded nature of the vascular benefit due to questionable loading strategies, a significant bleeding disadvantage, and potential cancer signals growing over time after prasugrel.
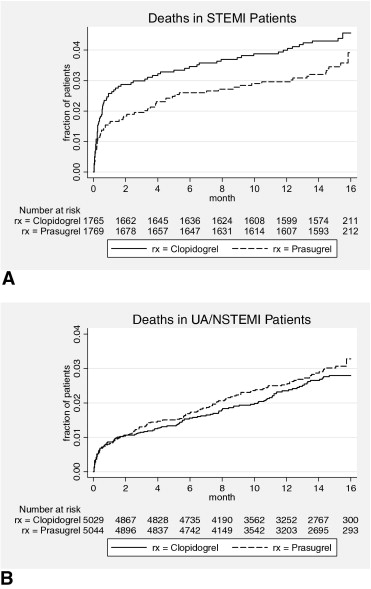
Recently, after approval with a “black box” warning, the FDA posted the complete documentation set (action package), revealing important additional information on prasugrel development, including associated mortality. The action package for prasugrel is a huge document (>900 pages), and it will be a challenge for practicing cardiologists to retrieve valuable information from its content. However, the differences in mortality are important to emphasize, because the FDA provided the additional analysis on the basis of the its prasugrel secondary report, in which an excess of death as a first reported event after prasugrel in the unstable angina (UA) or non–ST elevation myocardial infarction (NSTEMI) TRITON cohort was reported. Overall, although cardiovascular deaths trended slightly in favor of prasugrel (133 vs 150), an excess in bleeding fatalities (21 vs 5), and 4 additional cancer-related deaths after prasugrel diluted the mortality benefit almost completely. Moreover, final FDA notes revealed more deaths with unidentified causes after prasugrel (5 vs 2) in patients lost to follow-up without achieving primary end points but affecting all-cause mortality. In addition, the FDA provided a detailed distribution of deaths in TRITON, dependent on the type of qualifying initial occlusive event. The differences in mortality between patients with ST elevation myocardial infarctions (STEMIs) and those with UA or NSTEMIs are presented in Figure 1 .