Patients with coronary artery disease may develop not only ischemic events but also heart failure and death due to previous myocardial damage. The purpose of this study was to test the prognostic value of a panel of plasma biomarkers related to vascular (monocyte chemoattractant protein-1 [MCP-1] and soluble tumor necrosis factor-like weak inducer of apoptosis) and myocardial damage (galectin-3, N-terminal fragment of brain natriuretic peptide [NT-proBNP], and neutrophil gelatinase-associated lipocalin) in 706 patients with chronic coronary artery disease followed for 2.2 ± 0.99 years. Secondary outcomes were the incidence of acute ischemic events (ST elevation myocardial infarction, non-ST elevation acute coronary syndrome, stroke, or transient ischemic attack) and death or heart failure. The primary outcome was the combination of the secondary outcomes. Cox proportional hazards model was used for analysis. Fifty-three patients developed acute ischemic events. Increasing MCP-1 plasma levels (p = 0.002), age, and body mass index predicted this outcome independently. Thirty-three patients developed death and/or heart failure. Galectin-3 (p = 0.007), NT-proBNP plasma levels (p = 0.004), hypertension, glomerular filtration rate, and the use of nitrates and anticoagulants were associated with this outcome independently. The development of the primary outcome was predicted independently by MCP-1 (p <0.001), NT-proBNP (p = 0.005), and galectin-3 (p = 0.019); hypertension; atrial fibrillation; and treatment with nitrates. Every biomarker with a value above the median increased the risk of developing this outcome by 1.832 (95% confidence interval 1.356 to 2.474, p <0.001). High-sensitivity C-reactive protein and lipid levels were not associated with any outcome. In conclusion, increasing MCP-1, galectin-3, and NT-proBNP plasma levels are associated with a greater incidence of cardiovascular events.
We have followed 711 patients with coronary artery disease, testing a panel of biomarkers including monocyte chemoattractant protein-1 (MCP-1) and soluble tumor necrosis factor-like weak inducer of apoptosis (sTWEAK), both involved in inflammation and atherothrombosis, among other processes ; galectin-3, related to heart failure, thrombosis, renal dysfunction, and even malignancies ; neutrophil gelatinase-associated lipocalin (NGAL) involved in neutrophil activation, heart failure, and renal damage ; and the inactive N-terminal fragment of brain natriuretic peptide (NT-proBNP), related mainly to heart failure. High-sensitivity C-reactive protein was studied as a reference, given the large amount of information published with this biomarker.
Methods
The research protocol complies with the Declaration of Helsinki and was approved by the ethics committees of the participant hospitals. All patients signed informed consent documents. The Biomarkers in Acute Coronary Syndrome (BACS) and Biomarkers in Acute Myocardial Infarction (BAMI) studies included patients admitted at the hospitals Fundación Jiménez Díaz, Fuenlabrada, Móstoles, and Alcorcón in Madrid with either non-ST elevation acute coronary syndrome (NSTEACS) or ST elevation myocardial infarction (STEMI). NSTEACS was defined as angina at rest lasting >20 minutes in the previous 24 hours, or new-onset class III to IV angina, along with transient ST depression or T-wave inversion on the electrocardiogram considered diagnostic by the attending cardiologist, and/or troponin elevation. STEMI was defined as symptoms compatible with angina lasting >20 minutes, ST elevation in 2 adjacent leads on the electrocardiogram without response to nitroglycerin, and troponin elevation. Exclusion criteria were age >85 years, coexistence of other significant cardiac disorders except left ventricular hypertrophy secondary to hypertension, coexistence of any illness or toxic habits that could limit patient survival, impossibility to perform revascularization when indicated, and subjects in whom follow-up was not possible. To avoid variability of findings due to excessive heterogeneity in the intervals between the acute event and blood extraction, the investigators agreed to exclude patients who were not clinically stable at day 6 of the index event.
In addition to plasma withdrawal at discharge, a second plasma sample was extracted from 6 to 12 months later, on an outpatient basis. This study reports data from the clinical and analytical findings obtained at this second plasma extraction, relating them to subsequent follow-up.
From July 2006 to April 2010, a total of 1,898 patients were discharged from the hospitals of the study with a diagnosis of NSTEACS or STEMI. Of them, 711 participated in this substudy initiated from 6 to 12 months after the acute event ( Figure 1 ). The first visit took place from January 2007 to February 2011. The last follow-up visits were performed in May 2012.
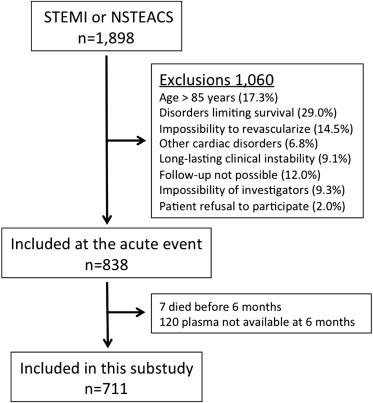
As there was no previous information on the panel of biomarkers studied, sample size calculation was not possible. At baseline, a complete set of clinical variables of the patients was recorded and 12-hour fasting venous blood samples were withdrawn and collected in ethylenediaminetetraacetic acid. Blood samples were centrifuged at 2,500 g for 10 minutes and plasma was stored at −80°C. Patients were seen every year at their hospital. At the end of follow-up (maximum 4.6 years), the medical records were reviewed and the patient status was confirmed by telephone contact.
The secondary outcomes were (1) recurrence of acute ischemic events such as NSTEACS, STEMI, stroke, or transient ischemic attack and (2) incidence of heart failure or death. The primary outcome was the composite of the secondary end points. NSTEACS and acute STEMI were defined as explained previously. A past myocardial infarction was diagnosed when new pathologic Q waves were present on the electrocardiogram along with a new myocardial scar identified by either echocardiography or nuclear magnetic resonance imaging in the same myocardial region. Stroke was defined as the rapid onset of a persistent neurologic deficit attributable to a focal vascular cause lasting >24 hours or a transient neurologic deficit accompanied by new ischemic lesions at imaging studies. A transient ischemic attack was defined as a transient stroke with signs and symptoms resolving before 24 hours, in the absence of acute ischemic lesions at imaging techniques. Events were adjudicated by at least 2 investigators of the study, along with a neurologist for cerebrovascular events. The heart failure end point was assigned to patients hospitalized for this reason.
The investigators who performed the analytical studies (LB-C, DA, OL, and JLM-V) were unaware of clinical data. Plasma concentrations of MCP-1, galectin-3, sTWEAK, and NGAL were determined in duplicate using commercially available enzyme-linked immunosorbent assay kits (BMS279/2, Bender MedSystems, Burlingame, California; DCP00, R&D Systems, Minneapolis, Minnesota; BMS2006INST, Bender MedSystems, Burlingame, California; and Kit 036, BioPorto, Gentofte, Denmark, respectively) following the manufacturers’ instructions. Intra- and interassay coefficients of variation were 4.6% and 5.9% for MCP-1, 6.2% and 8.3% for galectin-3, 6.1% and 8.1% for sTWEAK, and 5.3% and 7.9% for NGAL, respectively. High-sensitivity C-reactive protein was assessed by latex-enhanced immunoturbidimetry (ADVIA 2400 Chemistry System; Siemens, Munich, Germany) and NT-proBNP by immunoassay (VITROS; Ortho Clinical Diagnostics Raritan, New Jersey). Lipid, glucose, and creatinine levels were determined by standard methods (ADVIA 2400 Chemistry System; Siemens).
Quantitative data that followed a normal distribution are presented as mean ± SD and were compared using the Student t test. Those not normally distributed are displayed as median (interquartile range) and were compared using the Mann-Whitney test. Qualitative variables are displayed as percentages and were compared using chi-square or Fisher’s exact test when appropriate. Cox proportional hazards model was used with forward stepwise selection to assess the variables associated with the primary and the secondary outcomes. Variables with p <0.05 were entered into the model and those with p >0.10 were removed. In model 1, risk was estimated including age, gender, diabetes, smoking status, hypertension, body mass index, and lipid levels. In model 2, relevant clinical, analytical, and treatment variables used in the clinical practice were added: history of peripheral artery disease, cerebrovascular events, or atrial fibrillation; ejection fraction <40%, glomerular filtration rate assessed by Chronic Kidney Disease Epidemiology Collaboration method, high-sensitivity C-reactive protein, therapy with aspirin, clopidogrel, statins, acenocumarol, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, β blockers, nitrates and/or nitroglycerin, and diuretics; and type of last acute coronary event, number of diseased vessels, use of percutaneous revascularization, drug-eluting stents, and existence of complete revascularization at that event. Finally, in model 3, the biomarkers studied were included. Kaplan-Meier curves and log-rank test were used to compare time to outcome according to a multimarker score. Analyses were performed with SPSS 19.0 (IBM Corp., Armonk, New York).
Results
Mean follow-up period was 2.2 ± 0.99 years. Five patients were lost to follow-up, yielding 706 cases for analysis. Time from the last acute coronary event until plasma withdrawal and the beginning of follow-up was 7.5 ± 3.0 months. During this event, 97.9% of patients underwent coronary angiography.
Fifty-three patients met the secondary outcome of development of acute ischemic events. There were 4 episodes of STEMI, 22 of non-STEMI, 17 of unstable angina, 8 strokes, and 10 transient ischemic attacks. Four patients experienced 2 events and 2 patients experienced 3 events.
Patients who developed this outcome showed increased MCP-1 (165.6 [124.1 to 194.9] vs 128.8 [103.1 to 163.8] pg/ml, p <0.001), galectin-3 (8.89 [6.96 to 11.41] vs 7.73 [5.94 to 9.70] ng/ml, p = 0.006) and NT-proBNP (261.0 [138.5 to 674.5] vs 163.0 [86.7 to 395.5] pg/ml, p = 0.005) plasma levels compared with those who did not. Also, increased age and body mass index, decreased glomerular filtration rate, greater prevalence of hypertension, female gender, and therapy with nitrates were present in patients developing this outcome. The remaining variables, including NGAL, sTWEAK, and high-sensitivity C-reactive protein, did not show significant differences.
By multivariate analysis, only MCP-1 plasma levels (hazard ratio 1.442, 95% confidence interval [CI] 1.144 to 1.817 per increment of 100 pg/ml, p = 0.002) remained as independent predictors of this end point along with age (hazard ratio 1.031, 95% CI 1.003 to 1.059, p = 0.027) and body mass index (hazard ratio 1.088, 95% CI 1.027 to 1.153, p = 0.004) in model 3.
Thirty-three patients developed the secondary outcome of heart failure or death. There were 16 episodes of heart failure and 23 deaths, with 6 patients experiencing 2 events. Nine deaths were due to cardiovascular causes and 4 due to malignancies. Infection, renal failure, bowel ischemia, gastrointestinal bleeding, and pancreatitis accounted for 1 death each. Five deaths were of unknown cause. Twenty-six diagnoses of cancer were made during follow-up.
MCP-1 (175.4 [143.1 to 210.2] vs 129.2 [103.6 to 165.1] pg/ml), galectin-3 (10.78 [7.87 to 14.47] vs 7.73 [5.94 to 9.61] ng/ml), NT-proBNP (1,300 [477 to 3,370] vs 163.5 [86.1 to 379.5] pg/ml), NGAL (262.8 [185.2 to 350.3] vs 165.4 [123.4 to 220.2] ng/ml; p <0.001 for all), and high-sensitivity C-reactive protein (3.87 [0.92 to 8.30] vs 1.92 [0.83 to 3.90] mg/L, p = 0.020) plasma levels were higher in patients who developed this outcome compared with those who did not. Moreover, among other features, these patients were older, had a lower glomerular filtration rate, showed a greater prevalence of diabetes, hypertension, peripheral artery disease, cerebrovascular accidents, atrial fibrillation, and ejection fraction <40%, and were taking acenocumarol and nitrates more often.
On multivariate analysis, NT-proBNP and galectin-3 were independent predictors of developing this outcome along with hypertension, therapy with nitrates and acenocumarol, and a low glomerular filtration rate ( Table 1 ).
| Variable | Model 1 | Model 2 | Model 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | p | Hazard Ratio | 95% CI | p | Hazard Ratio | 95% CI | p | ||||
| Inferior | Superior | Inferior | Superior | Inferior | Superior | |||||||
| Age | 1.110 | 1.064 | 1.158 | <0.001 | – | – | – | – | – | – | – | – |
| Diabetes | 2.661 | 1.279 | 5.534 | 0.009 | – | – | – | – | – | – | – | – |
| Hypertension | 7.374 | 0.996 | 54.608 | 0.050 | 6.460 | 0.848 | 49.232 | 0.072 | 7.824 | 1.034 | 59.203 | 0.046 |
| Stroke | – | – | – | – | 2.791 | 1.006 | 7.743 | 0.049 | – | – | – | – |
| Atrial fibrillation | – | – | – | – | 3.715 | 1.619 | 8.526 | 0.002 | – | – | – | – |
| Nitrates | – | – | – | – | 4.648 | 2.161 | 9.995 | <0.001 | 4.102 | 1.901 | 8.851 | <0.001 |
| Glomerular filtration rate | – | – | – | – | 0.955 | 0.935 | 0.976 | <0.001 | 0.965 | 0.942 | 0.988 | 0.003 |
| Acenocumarol | – | – | – | – | – | – | – | – | 3.064 | 1.323 | 7.098 | 0.009 |
| NT-proBNP | – | – | – | – | – | – | – | – | 1.025 ∗ | 1.008 ∗ | 1.042 ∗ | 0.004 |
| Galectin-3 | – | – | – | – | – | – | – | – | 1.071 | 1.019 | 1.125 | 0.007 |
Seventy-eight patients developed the primary outcome of acute ischemic events, heart failure, or death. Twelve patients developed 2 events and 5 patients experienced 3 events.
Patients who met this end point showed increased MCP-1, galectin-3, and NGAL plasma levels compared with those who remained stable, whereas sTWEAK and high-sensitivity C-reactive protein plasma levels did not differ between both groups. ( Table 2 lists differences in baseline variables.)
| Variable | Acute Ischemic Event, Heart Failure, or Death | p Value | |
|---|---|---|---|
| No (n = 628), % | Yes (n = 78), % | ||
| Age (yrs) | 59.0 (51.0-71.0) | 72.0 (61.8-78.0) | <0.001 |
| Men | 77 | 63 | 0.007 |
| White | 97 | 97 | 0.584 |
| Body mass index (kg/m 2 ) | 28.4 (25.8-30.8) | 29.0 (25.7-34.1) | 0.012 |
| Diabetes mellitus | 22 | 33 | 0.020 |
| Present smoker | 6.7 | 5.1 | 0.409 |
| Hypertension | 62 | 89 | <0.001 |
| Peripheral artery disease | 3.3 | 7.7 | 0.066 |
| Cerebrovascular events | 3.0 | 6.4 | 0.114 |
| Previous coronary artery bypass graft | 7.3 | 16.7 | 0.008 |
| Previous percutaneous intervention | 78 | 78 | 0.564 |
| Atrial fibrillation | 3.3 | 16.7 | <0.001 |
| Ejection fraction <40% | 11.8 | 12.8 | 0.017 |
| Aspirin | 93 | 86 | 0.039 |
| Clopidogrel | 69 | 62 | 0.135 |
| Acenocumarol | 5 | 17 | <0.001 |
| Statins | 89 | 78 | 0.010 |
| Angiotensin-converting enzyme inhibitors | 56 | 47 | 0.089 |
| Angiotensin receptor blockers | 16 | 23 | 0.068 |
| β Blockers | 77 | 69 | 0.079 |
| Nitrates/nitroglycerin | 15 | 37 | <0.001 |
| Diuretics | 18 | 33 | 0.002 |
| STEMI/NSTEACS ∗ | 40/60 | 31/69 | 0.080 |
| Number of narrowed coronary arteries ∗ | 1.35 ± 0.80 | 1.57 ± 0.82 | 0.027 |
| Percutaneous intervention ∗ | 75 | 67 | 0.135 |
| Drug-eluting stent ∗ | 48 | 37 | 0.072 |
| Complete revascularization ∗ | 67 | 50 | 0.010 |
| LDL cholesterol (mg/dl) | 82.7 ± 24.2 | 86.9 ± 35.0 | 0.307 |
| HDL cholesterol (mg/dl) | 43.7 ± 10.7 | 45.3 ± 12.2 | 0.203 |
| Triglycerides (mg/dl) | 130.3 ± 84.6 | 135.9 ± 66.7 | 0.573 |
| Glomerular filtration rate (ml/min/1.73 m 2 ) | 79.3 (64.6-90.6) | 66.3 (47.1-83.0) | <0.001 |
| High-sensitivity C-reactive protein (mg/L) | 4.41 ± 9.73 | 5.20 ± 9.05 | 0.495 |
| MCP-1 (pg/ml) | 127.1 (102.2-160.3) | 166.8 (131.3-197.9) | <0.001 |
| Galectin-3 (ng/ml) | 7.70 (5.91-9.50) | 9.40 (7.01-12.03) | <0.001 |
| NT-proBNP (pg/ml) | 160 (85-359) | 477 (167-1,158) | <0.001 |
| NGAL (ng/ml) | 164.9 (124.0-219.1) | 210.4 (131.1-275.5) | 0.002 |
| sTWEAK (pg/ml) | 221.9 ± 135.4 | 213.2 ± 144.9 | 0.595 |
∗ Clinical data at the acute coronary event 6 to 12 months before plasma extraction and the start of follow-up.
On multivariate analysis, increasing MCP-1, galectin-3, and NT-proBNP levels were independent predictors of the occurrence of this end point along with hypertension, atrial fibrillation, and nitrate therapy ( Table 3 ). As expected, patients on nitrates displayed a worse clinical profile than those not on this therapy (not shown).
| Variable | Model 1 | Model 2 | Model 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | p | Hazard Ratio | 95% CI | p | Hazard Ratio | 95% CI | p | ||||
| Inferior | Superior | Inferior | Superior | Inferior | Superior | |||||||
| Age | 1.061 | 1.037 | 1.084 | <0.001 | 1.030 | 1.003 | 1.059 | 0.033 | – | – | – | – |
| Hypertension | 2.594 | 1.278 | 5.265 | 0.008 | 2.321 | 1.126 | 4.786 | 0.023 | 2.973 | 1.461 | 6.049 | 0.003 |
| Body mass index | 1.085 | 1.034 | 1.140 | 0.001 | 1.071 | 1.017 | 1.127 | 0.009 | – | – | – | – |
| Atrial fibrillation | – | – | – | – | 2.045 | 1.084 | 3.859 | 0.027 | 2.494 | 1.312 | 4.741 | 0.005 |
| Nitrates | – | – | – | – | 2.367 | 1.435 | 3.903 | 0.001 | 2.471 | 1.498 | 4.076 | <0.001 |
| Glomerular filtration rate | – | – | – | – | 0.983 | 0.968 | 0.999 | 0.032 | – | – | – | – |
| MCP-1 | – | – | – | – | – | – | – | – | 1.498 ∗ | 1.236 ∗ | 1.815 ∗ | <0.001 |
| NT-proBNP | – | – | – | – | – | – | – | – | 1.020 ∗ | 1.006 ∗ | 1.034 ∗ | 0.005 |
| Galectin-3 | – | – | – | – | – | – | – | – | 1.045 | 1.007 | 1.084 | 0.019 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


