A systematic review and meta-analysis was performed to evaluate the effects of carvedilol versus metoprolol on the incidence of postoperative atrial fibrillation in patients undergoing coronary artery bypass grafting in randomized controlled trials. Ovid MEDLINE, PubMed, CENTRAL, and Excepta Medica (EMBASE) were searched up to March 2013 for suitable randomized controlled trials. Data were pooled using random-effects model for pairwise analyses. A total of 4 trials with 601 patients were included in this analysis. Pairwise analyses showed that compared with metoprolol, carvedilol significantly reduced the incidence of postoperative atrial fibrillation (odds ratio 0.50, 95% confidence interval 0.32 to 0.80). In conclusion, compared with metoprolol, carvedilol significantly reduces the incidence of postoperative atrial fibrillation in patients undergoing coronary artery bypass grafting.
Although many approaches have been attempted to prevent postoperative atrial fibrillation, current guidelines suggest β blockers as first-line medication to prevent postoperative atrial fibrillation after coronary artery bypass graft. However, pharmacologic characteristics differ considerably between β blockers. Carvedilol is a unique β blocker, exerting vasodilating, antioxidant, and free-radical scavenging effects. Although metoprolol preferentially inhibits cardiac β1 receptors as opposed to β2 receptors, carvedilol inhibits postsynaptic cardiac β1, β2, and α1 receptors and presynaptic β2 receptors, upregulates cardiac muscarinic M2 receptors, and evokes antioxidant effects, properties that are not shared by atenolol, metoprolol, or bisoprolol. Thus, we sought to perform a systematic review and meta-analysis of randomized controlled trials to determine if carvedilol reduces the incidence of postoperative atrial fibrillation compared with metoprolol in patients undergoing coronary artery bypass grafting.
Methods
A systematic review of the available published reports according to the PRISMA guidelines for the conduct of systematic reviews of intervention studies was performed.
Studies were identified through searches of the following sources: Ovid MEDLINE (1997 to 2013), PubMed (1965 to 2013), EMBASE (1997 to 2013), and the Cochrane Central Register of Controlled Trials (CENTRAL). To identify further potentially relevant studies missed by the electronic database search, reference lists from identified trials and review articles were manually screened. Searches were updated using automated weekly e-mail alerts until March 2013. Full details of the search strategies and excluded trials are available as appendices by request.
Studies were selected for inclusion on the basis of the following criteria: study design: randomized controlled trials; types of participants: adults (≥18 years); intervention: carvedilol; comparator: metoprolol; and outcomes: incidence of postoperative atrial fibrillation, electrical cardioversion, length of stay, intensive care unit stay, all-cause mortality, and cardiovascular events (stroke or myocardial infarction). The titles and abstracts of studies identified by the search strategy were independently screened by 2 reviewers (CJB and ARM), and clearly irrelevant studies were discarded. Data extraction was conducted by mutual agreement, and all potential disagreement was solved by another investigator (JJD).
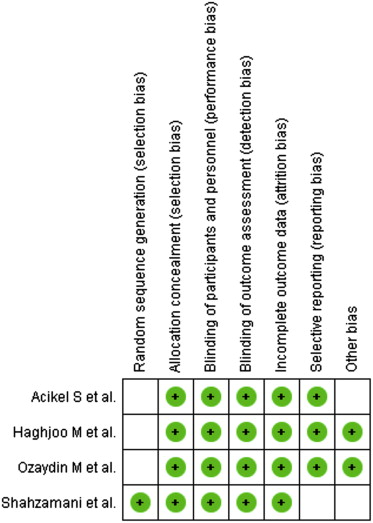
The following data elements were extracted from each study: the number of patients per arm, the nature of the intervention, patient inclusion criteria, percent men, timing of β-blocker administration, follow-up (days), definition of atrial fibrillation, atrial fibrillation measurement, and quality of study ( Tables 1 and 2 ). The following outcomes were also extracted from each trial: incidence of postoperative atrial fibrillation, direct current cardioversion, length of stay, intensive care unit stay, all-cause mortality, and cardiovascular events (stroke and myocardial infarction). Risk of bias was assessed using the criteria recommended by the Cochrane Collaboration, specifically evaluating sequence generation of allocation; allocation concealment; blinding of participants, staff, and outcome assessors; incomplete outcome data; selective outcome reporting; and other sources of bias ( Figure 1 ). Trials with high or unclear risk of bias for the first 3 criteria were considered as high–bias risk trials and the rest as low–bias risk trials ( Table 2 ). The data extraction was performed by 2 independent reviewers (CJB and ARM), and quality assessment was undertaken using standardized proforma by 2 reviewers (CJB and ARM). When decisions of study selection and/or data extraction conflicted, other investigators were involved to resolve the discrepancy.
| Trial | Year | n | % Men | β Blocker, Timing (Days) | Follow-Up (Days), Postop |
|---|---|---|---|---|---|
| Shahzamani et al | 2011 | 60 | 63.3 | 1 ∗ | 180 |
| Haghjoo et al | 2007 | 120 | 52.5 | 10 † | 5 |
| Acikel et al | 2007 | 110 | 71.8 | 3 † | 3 |
| Ozaydin et al | 2012 | 311 | 72.2 | ≥7 † | 10–12 |
∗ β Blocker started postoperatively.
| Trial | Inclusion Criteria | Atrial Fibrillation Measurement |
|---|---|---|
| Shahzamani et al | Elective coronary artery bypass graft | Continuous ECG |
| Haghjoo et al | Scheduled for first on-pump coronary artery bypass graft | Continuous ECG |
| Acikel et al | Elective coronary artery bypass graft | Continuous ECG |
| Ozaydin et al | Elective coronary artery bypass graft | 12-Lead ECG every morning |
We expressed outcome results for each study as odds ratios (OR) and 95% confidence intervals (CIs). Summary estimates were computed using a DerSimonian and Laird random-effects model for pairwise analyses. Heterogeneity was assessed using the I 2 statistic, defined as the proportion of total variation observed between the trials attributable to differences between trials rather than sampling error (chance) with values <25% considered as low and >75% as high. RevMan 5 was used for all analyses.
Results
The literature search yielded 850 titles; of which, 8 were reviewed in full text on the basis of the inclusion criteria ( Figure 2 ). Of these, 4 studies were deemed eligible for inclusion. Table 1 summarizes the characteristics of the included studies.
All trials were randomized controlled trials of carvedilol compared with metoprolol in patients undergoing coronary artery bypass surgery. All background medications and baseline characteristics were statistically similar between the comparison groups in each trial.
Four trials (n = 601) reported on the incidence of postoperative atrial fibrillation. Compared with metoprolol, carvedilol significantly reduced the incidence of postoperative atrial fibrillation (OR 0.50, 95% CI 0.32 to 0.80, I 2 = 15%; Figure 3 ). Two trials (n = 230) reported on direct current cardioversion, disclosing no difference in direct current cardioversion between carvedilol and metoprolol (OR 0.32, 95% CI 0.03 to 3.19; Figure 4 ). Four trials (n = 497) reported on all-cause mortality, showing no superiority of carvedilol versus metoprolol (OR 0.44, 95% CI 0.10 to 2.03, I 2 = 0%; Figure 5 ). The same 4 trials reported on the combined end point of all-cause mortality, myocardial infarction, or stroke ( Figure 6 ), which did not indicate that carvedilol showed a benefit compared with metoprolol (overall OR 0.49, 95% CI 0.17 to 1.42), versus metoprolol succinate (OR 0.44, 95% CI 0.13 to 1.47), and versus metoprolol tartrate (OR 0.72, 95% CI 0.05 to 11.17). The risk of nonfatal myocardial infarction or stroke was not significantly less with carvedilol than with metoprolol (OR 0.60, 95% CI 0.17 to 2.19, I 2 = 0%).




