Coronary CT angiography (CCTA) is used in patients with low-intermediate chest pain presenting to the emergency department for its reliability in excluding acute coronary syndrome (ACS). However, its influence on medication modification in this setting is unclear. We sought to determine whether knowledge of CCTA-based coronary artery disease (CAD) was associated with change in statin and aspirin prescription. We used the CCTA arm of the Rule Out Myocardial Infarction using Computed Angiographic Tomography II multicenter, randomized control trial (R-II) and comparison cohort from the observational Rule Out Myocardial Infarction using Computed Angiographic Tomography I cohort (R-I). In R-II, subjects were randomly assigned to CCTA to guide decision making, whereas in R-I patients underwent CCTA with results blinded to caregivers and managed according to standard care. Our final cohort consisted of 277 subjects from R-I and 370 from R-II. ACS rate was similar (6.9% vs 6.2% respectively, p = 0.75). For subjects with CCTA-detected obstructive CAD without ACS, initiation of statin was significantly greater after disclosure of CCTA results (0% in R-I vs 20% in R-II, p = 0.009). Conversely, for subjects without CCTA-detected CAD, aspirin prescription was lower with disclosure of CCTA results (16% in R-I vs 4.8% in R-II, p = 0.001). However, only 68% of subjects in R-II with obstructive CAD were discharged on statin and 65% on aspirin. In conclusion, physician knowledge of CCTA results leads to improved alignment of aspirin and statin with the presence and severity of CAD although still many patients with CCTA-detected CAD are not discharged on aspirin or statin. Our findings suggest opportunity for practice improvement when CCTA is performed in the emergency department.
The presence and severity of coronary CT angiography (CCTA)–detected coronary artery disease (CAD) provides prognostic value for cardiovascular events and mortality. Despite this strong association between CAD detected by CCTA and outcomes, there is limited evidence on whether CCTA findings can influence the prescription of cardiovascular preventive medical therapies such as aspirin and statin. Using both the observational Rule Out Myocardial Infarction using Computed Angiographic Tomography (ROMICAT) I cohort in which subjects at low-intermediate risk presenting with acute chest pain underwent CCTA in the emergency department (ED) with results blinded to caretakers and the multicenter ROMICAT II cohort with similar inclusion criteria but with CCTA results disclosed to providers, we sought to determine whether physician knowledge of CCTA results would result in better alignment of medical therapy prescription with the presence of CAD on CCTA.
Methods
We used data from 2 trials that included subjects at low-intermediate risk presenting with acute chest pain to the ED and suspicion for acute coronary syndrome (ACS) : (1) the observational ROMICAT I (R-I) study in which 368 subjects were managed according to standard care but underwent CCTA with results blinded to health care providers and (2) the multicenter, randomized controlled ROMICAT II (R-II) trial in which 501 of the enrolled 1,000 subjects were randomized to undergo CCTA, the results of which were disclosed to care providers. Thus, the 2 studies differed as to whether CCTA results were disclosed to care providers. Our institutional review board approved these study protocols and all patients provided informed consent.
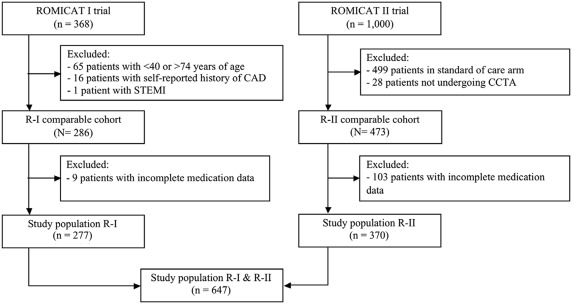
Although the trials’ inclusion criteria were very similar, there were some differences in exclusion criteria. Therefore, to create comparable cohorts, we excluded subjects from the R-I trial that would have met exclusion criteria for the R-II trial. More precisely, we excluded patients who were aged <40 years or >74 years (n = 65), and those who had a self-reported history of CAD (n = 17). See flow diagram ( Figure 1 ). In the R-II cohort, we excluded 28 subjects who were randomized to the CCTA arm but did not undergo CCTA. Finally, we excluded subjects with incomplete medication data (9 subjects in R-I and 103 subjects in R-II). Thus, the final study population consisted of 277 subjects from R-I and 370 subjects from R-II.
Two physicians blinded to individual patient CCTA results performed medication data collection. Admission and discharge medication prescription data were collected for each patient by systematically reviewing medical records during the index ED visit and hospitalization, including ED admission and discharge notes, as well as inpatient admission and discharge summary if the patient was admitted to the hospital (143 subjects in R-I and 82 subjects in R-II). Contraindication to aspirin (aspirin) therapy was defined as a severe aspirin allergy (anaphylaxis, angioedema, and so forth) or documented intolerance due to a history of major gastrointestinal bleed (requiring transfusion) or intracranial bleed. Contraindication to statin therapy was defined as a history of statin-induced myopathy, rhabdomyolysis, or other documented statin intolerance.
Risk factors were assessed at the time of subject enrollment on the basis of self-report and review of medical records for the index hospitalization. Hypertension was defined as a systolic blood pressure of at least 140 mm Hg or diastolic blood pressure of at least 90 mm Hg or current antihypertensive treatment. Diabetes mellitus was defined as a fasting plasma glucose ≥126 or treatment with a hypoglycemic agent. Peripheral vascular disease was defined as having a history of transient ischemic attack, cerebrovascular event, or peripheral arterial disease. Family history of CAD was defined as having a first degree women (<65 years of age) or men (<55 years of age) relative with a documented history of myocardial infarction or sudden cardiac death. Subjects were classified as smokers if they had smoked at least 1 cigarette per day in the year before the study. Lipid profiles were collected as part of the study protocol in R-I subjects at the time of admission, and collected retrospectively in R-II subjects (admission laboratory data or most recent lipid panel within 1 year before admission). The 10-year Framingham Risk Score was calculated for each patient, and patients were stratified into the following 3 groups: <10%, 10% to 20%, and >20% for 10-year risk of a cardiovascular event. The definition of ACS during the index hospitalization in both the R-I and R-II cohorts have been published previously. Briefly, in both trials, ACS was defined as either an acute myocardial infarction or unstable angina pectoris. Establishment of this diagnosis was based on an outcome panel of experienced physicians who reviewed prospectively collected patient clinical information and medical records during the index hospitalization. The outcome panel was blinded to the findings of CCTA, and disagreement was resolved by the adjudication of an additional cardiologist.
Based on CCTA results, patients were categorized into having no CAD, nonobstructive CAD (most severe stenosis <50%), or obstructive CAD (most severe stenosis ≥50% or inconclusive results). In the R-I cohort, this analysis was performed in a core laboratory, whereas in the multicenter R-II trial, we relied on individual site interpretation data to evaluate the influence of CCTA results on medication prescription.
Baseline demographics of the 2 patient cohorts were compared with the use of independent sample t test for continuous variables, Fisher’s exact test for categorical variables, and the Wilcoxon rank-sum test for ordinal variables. Continuous variables are expressed as mean ± SD; categorical variables are described by frequency. Stepwise logistic regression analysis was used to assess the association between disclosure of CCTA results (R-II cohort) and changes in medical therapy, controlling for confounding baseline variables. All analyses were performed using Stata, version 13.1, (StataCorp, College Station, Texas). For all analyses, a 2-tailed p value <0.05 was required to reject the null hypothesis.
Results
Baseline characteristics of our R-I (52.3 ± 7.9 years, 58% men) and R-II (53.8 ± 8.0 years, 53% men) comparable cohorts are listed in Table 1 . Overall clinical characteristics were similar between the cohorts. Furthermore, there was a higher prevalence of diabetes and hypertension in the R-II cohort, whereas mean total cholesterol and low-density lipoprotein (LDL)–cholesterol were greater in R-I. There were no significant differences in Framingham Risk category and ACS rate between R-I and R-II.
| Variable | R-I (N = 277) | R-II (N = 370) | P value |
|---|---|---|---|
| Age (years), mean ± SD | 52.3 ± 7.9 | 53.8 ± 8.0 | 0.026 |
| Men | 161 (58%) | 196 (53%) | 0.20 |
| Hypertension | 105 (38%) | 194 (52%) | <0.001 |
| Diabetes mellitus | 29 (11%) | 64 (17%) | 0.017 |
| Dyslipidemia | 105 (38%) | 163 (44%) | 0.13 |
| Former or current smoker | 141 (51%) | 183 (50%) | 0.75 |
| Family history of premature CAD | 74 (27%) | 98 (27%) | 1.00 |
| Cholesterol (mg/dl), mean ± SD | |||
| Total | 200 ± 40 | 188 ± 39 | <0.001 |
| High-Density Lipoprotein | 52 ± 15 | 50 ± 18 | 0.41 |
| Low-Density Lipoprotein | 118 ± 36 | 108 ± 37 | 0.003 |
| Framingham Risk | (N = 252) | (N = 243) | 0.91 |
| <10% | 191 (76%) | 182 (75%) | |
| 10%-20% | 48 (19%) | 46 (19%) | |
| >20% | 13 (5.2%) | 15 (6.2%) | |
| Acute Coronary Syndrome | 19 (6.9%) | 23 (6.2%) | 0.75 |
Complete admission and discharge prescription data for aspirin and statin were obtainable in 277 subjects in the R-I cohort and 370 subjects in the R-II cohort ( Figure 1 ). Of these, a total of 14 subjects were identified as having a contraindication to aspirin, and 14 subjects were identified as having a contraindication to statin (See Table 2 ). Overall, there was no significant difference in the overall change in aspirin or statin therapy from admission to discharge between the R-I and R-II cohorts. Most subjects who had a change in medical therapy from admission to discharge were “upgraded” as opposed to “downgraded”; in other words, they were not on aspirin or statin at admission but were prescribed the medication at discharge.
| R-I (N = 277) | R-II (N = 370) | P value | |
|---|---|---|---|
| Contraindication to Aspirin | 6 (2.2%) | 8 (2.2%) | 1.00 |
| Aspirin Therapy ∗ | N = 271 | N = 362 | |
| Admission Medication | 64 (24%) | 88 (24%) | 0.85 |
| Discharge Medication | 120 (44%) | 143 (40%) | 0.25 |
| Change in Medication | 58 (21%) | 59 (16%) | 0.10 |
| Upgrade in Therapy | 57 (21%) | 57 (16%) | 0.095 |
| Downgrade in Therapy | 1 (0.4%) | 2 (0.6%) | 1.00 |
| Contraindication to Statin | 9 (3.3%) | 5 (1.4%) | 0.11 |
| Statin Therapy ∗ | N = 268 | N = 365 | |
| Admission Medication | 62 (23%) | 112 (30.7%) | 0.038 |
| Discharge Medication | 76 (28%) | 145 (39.7%) | 0.003 |
| Change in Medication | 18 (6.7%) | 33 (9.0%) | 0.31 |
| Upgrade in Therapy | 16 (6.0%) | 33 (9.0%) | 0.18 |
| Downgrade in Therapy | 2 (0.8%) | 0 (0.0%) | 0.18 |
Overall, upgrade of statin therapy occurred in 9.0% of subjects (33 of 365) in R-II and 6.7% of subjects (18 of 268) in R-I. Although the change in statin therapy from admission to discharge was not significantly different between the 2 cohorts, there was a higher rate of statin therapy at both admission and discharge in the R-II cohort compared with the R-I cohort (admission: 31% vs 23%, p = 0.038; discharge: 40% vs 28%, p = 0.003). The distribution of CCTA-detected CAD findings is listed in Table 3 . Roughly half of the subjects in both cohorts had CCTA-detected CAD. Obstructive CAD was found in 16% of R-I and 25% of R-II subjects.
| R-I (N = 277) | R-II (N = 370) | P value | |
|---|---|---|---|
| Presence and Severity of CAD | 0.02 | ||
| No CAD | 144 (52%) | 172 (47%) | |
| Non-Obstructive CAD (<50%) | 89 (32%) | 106 (29%) | |
| Obstructive CAD (≥50% or indeterminate) | 44 (16%) | 92 (25%) | |
| CCTA Results Disclosed | No | Yes |
Statin therapy
In subjects with CCTA-detected obstructive CAD, 30% of subjects in the R-II cohort were started on statin therapy, whereas 23% of subjects in the R-I cohort were started on statin therapy ( Table 4 ). Stratifying the subjects with obstructive CAD by the presence or absence of clinical ACS unveiled a marked difference in statin prescription between the R-I and R-II cohorts ( Table 5 ). Specifically, subjects with obstructive CAD without ACS were significantly more likely to be prescribed statin therapy in R-II compared with R-I (upgrade to statin: 20% (14 of 69) vs 0% (0 of 27), p = 0.009). Similar findings were noted when only including subjects who were admitted to the hospital. There was no difference in rates of statin prescription in the subjects with both obstructive CAD and ACS. Of patients with no CAD and nonobstructive CAD, there were no significant differences between the R-I and R-II cohorts with respect to change in statin therapy.
| No CAD | Non-obstructive CAD | Obstructive CAD | |||||||
|---|---|---|---|---|---|---|---|---|---|
| R-I (N = 144) | R-II (N = 172) | p | R-I (N = 89) | R-II (N = 106) | p | R-I (N = 44) | R-II (N = 92) | p | |
| Aspirin ∗ | (N = 140) | (N = 168) | (N = 87) | (N = 102) | (N = 44) | (N = 92) | |||
| Admission Medication | 30 (21%) | 40 (24%) | 0.68 | 21 (24%) | 25 (25%) | 1.00 | 13 (30%) | 23 (25%) | 0.68 |
| Discharge Medication | 52 (37%) | 47 (28%) | 0.09 | 40 (46%) | 36 (35%) | 0.14 | 28 (64%) | 60 (65%) | 0.85 |
| Change in Medication | 24 (17%) | 9 (5.4%) | 0.001 | 19 (22%) | 11 (11%) | 0.046 | 15 (34%) | 39 (42%) | 0.45 |
| Upgrade in Therapy | 23 (16%) | 8 (4.8%) | 0.001 | 19 (21.8%) | 11 (11%) | 0.046 | 15 (34%) | 38 (41%) | 0.46 |
| Downgrade in Therapy | 1 (0.7%) | 1 (0.6%) | 1.00 | 0 (0.0%) | 0 (0.0%) | — | 0 (0.0%) | 1 (1.1%) | 1.00 |
| Statin ∗ | (N = 139) | (N = 172) | (N = 86) | (N = 102) | (N = 43) | (N = 91) | |||
| Admission Medication | 22 (16%) | 49 (29%) | 0.01 | 24 (28%) | 28 (28%) | 1.00 | 16 (37%) | 35 (39%) | 1.00 |
| Discharge Medication | 21 (15%) | 51 (30%) | 0.003 | 29 (33%) | 32 (31%) | 0.76 | 26 (61%) | 62 (68%) | 0.44 |
| Change in Medication | 3 (2.2%) | 2 (1.2%) | 0.66 | 5 (5.8%) | 4 (3.9%) | 0.73 | 10 (23%) | 27 (30%) | 0.54 |
| Upgrade in Therapy | 1 (0.7%) | 2 (1.2%) | 1.00 | 5 (5.8%) | 4 (3.9%) | 0.73 | 10 (23%) | 27 (30%) | 0.54 |
| Downgrade in Therapy | 2 (1.4%) | 0 (0.0%) | 0.20 | 0 (0.0%) | 0 (0.0%) | — | 0 (0.0%) | 0 (0.0%) | — |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


