Chapter 124
Upper Extremity Sympathectomy
Nelson Wolosker, Paulo Kauffman
The first studies on the anatomy of the autonomic nervous system are attributed to Galen in the 2nd century AD, but it was only in the last decade of the 19th century that the effects resulting from the blockade of preganglionic and postganglionic neurons were described by Langley.1 The first sympathectomy is attributed to Alexander in 1889, who tried to treat an epileptic patient by this surgical procedure.2 He was followed by Jonnesco, who in 1896 performed sympathectomy on a large number of epileptic patients.3 In 1899, Jaboulay resected the lower cervical chain in a patient with exophthalmia and goiter.4 Because of lack of success in the operative treatment of these conditions as well as of many others with no effective medical or surgical alternatives (migraine, renal pain, poliomyelitis), interest in intervention on the sympathetic nervous system waned for a time.
The first successful clinical application of sympathectomy occurred in patients with angina pectoris. The knowledge that afferent visceral fibers could transmit sensory impulses through the sympathetic chain to the central nervous system led Jonnesco in 1916 to successfully perform cervicothoracic sympathectomy for angina pectoris with the aim of pain suppression.5 The first to use cervical sympathectomy to treat a patient with hyperhidrosis was Kotzareff in 1920.6 In 1924, Hunter tried to use sympathectomy to reduce muscle tonus in patients with spastic paralysis; although this intervention did not show any beneficial effect on this condition, he observed a significant increase in circulation in denervated limbs.7 This led to its use in treating Raynaud’s disease and other types of vasospastic disease8. In 1924, Diez reported 100% success in treating 150 cases of thromboangiitis obliterans in the upper limbs.9
There was a technical evolution in open surgery involving different surgical approaches to resect the sympathetic ganglia: supraclavicular (cervical),10 axillary transthoracic,11 dorsal (posterior),12 dorsal midline,13 and anterior transthoracic.14 By the end of the 1930s, the main indications for cervicothoracic sympathectomy had started to be delineated: hyperhidrosis, thromboangiitis obliterans, and vasospastic conditions.
Using the development of thoracoscopy, introduced by Jacobaeus in 1910,15 Hughes in 1942 performed the first thoracoscopic sympathectomy.16 Kux, in 1953, was the first to publish a large experience with this method.17 However, despite the good results, for unknown reasons this technique did not achieve international acceptance for almost 30 years. In the 1980s, the endoscopic technique was used to perform sympathetic denervation in the upper limbs by a few groups of surgeons.18
In the 1990s, advances in optical systems and instruments for thoracoscopic surgery made it possible to use video-assisted thoracoscopy to perform sympathectomy.19 The low morbidity, good cosmetic results, decrease in incidence of Horner’s syndrome, and short hospital stay stimulated patients with hyperhidrosis to request their physicians to use video-assisted thoracoscopic sympathectomy (VATS).20 Because these patients are young and healthy and VATS is an elective procedure, there have been positive outcomes, and this type of surgery is being increasingly performed. During the last 15 years, thousands of operations have been reported, which has led to technical improvements and better results.
Anatomy
Sympathetic Ganglia
The motor sympathetic route is formed by three neurons (Fig. 124-1). The cell body of the first neuron is located in the sudomotor and vasomotor centers, mainly in the hypothalamus. Its axon projects along the dorsal longitudinal and spinovestibular fascicles to the cell body of the second neuron (preganglionic neuron), which is located in the intermediolateral nucleus of the spinal gray matter, between the first thoracic and the second lumbar vertebrae. Its axon (the preganglionic fiber) exits the medulla through the ventral root of the spinal nerves and, through the white communicating branch, projects to the paravertebral ganglion, where it forms a synapse with the cell body of the third neuron, the postganglionic neuron. Its axon (the postganglionic fiber) leaves the sympathetic chain through the gray communicating branch into the spinal nerve and is distributed peripherally. The ganglia are also interconnected longitudinally by axons from preganglionic neurons that run rostrally or caudad to the neighboring ganglia of the chains.
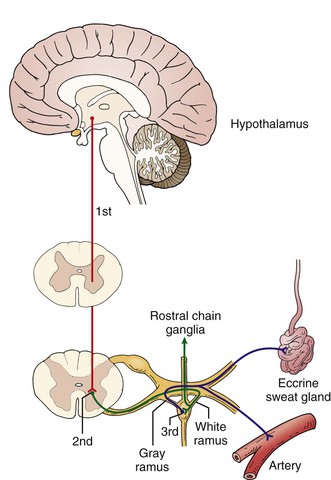
Figure 124-1 The motor sympathetic route. The cell body of the first neuron is located in the sudomotor and vasomotor centers, the second neuron (preganglionic neuron) is located in the intermediolateral nucleus of the spinal gray matter, and the third neuron (postganglionic neuron) is located in the paravertebral ganglia.
In the neck, there are normally three ganglia in the sympathetic chain. The superior cervical ganglion results from the fusion of the first four sympathetic cervical ganglia; it is located at the level of the transverse process of the second and third cervical vertebrae and supplies the head and neck. The middle cervical ganglion is located at the level of the sixth cervical vertebra. The inferior cervical ganglion is generally fused with the first thoracic ganglion (G1) to form the cervicothoracic ganglion (stellate ganglion), which is located anterior to the head of the first rib and covered by the pleura.
In the thoracic region, the ganglia of the sympathetic chain are positioned anteriorly to the transverse processes of the thoracic vertebrae and are covered by the parietal pleura. They are fewer in number than the spinal thoracic nerves because of the fusion of the first thoracic ganglion with the inferior cervical ganglion, fusion of the last thoracic ganglion with the first lumbar ganglion, and fusion of the thoracic ganglia with each other.
The greater, lesser, and least splanchnic nerves are formed by preganglionic fibers originating from the 5th to the 12th thoracic medullary segments. They cross the corresponding sympathetic ganglia without forming synapses with them and end in the celiac, aorticorenal, and superior and inferior mesenteric ganglia. The major splanchnic nerve plays a particularly important role in visceral pain because of the large numbers of visceral afferent fibers it contains. Hence, splanchnicectomy may be employed for treatment of unmanageable visceral pain, particularly in pancreatic diseases (cancer and pancreatitis).21 In the past, thoracolumbar sympathectomies were performed for treatment of hypertension, a technique currently abandoned with the advent of modern antihypertensive drugs.22
Sympathetic Innervation of the Upper Limbs
The preganglionic fibers responsible for the innervation of the upper limbs originate from the second to eighth thoracic medullary segments, most of them below the fourth segment. The fibers enter the paravertebral sympathetic chain through the white communicating branches of the corresponding ganglia and have an ascending pathway in which a synapse is formed with cells located in the second thoracic ganglion, the stellate ganglion, and probably the middle cervical ganglion.
It is of surgical interest that no preganglionic fibers enter the sympathetic chain above the first thoracic ganglion, which participates in innervation of the limb in only 10% of the cases.
In most patients, the thoracic sympathetic trunk is located in the middle of the intercostal space, on the bottom edge of the top rib or the top edge of the bottom rib.23–25 Therefore, when we section the sympathetic chain on two consecutive ribs, there is a high probability that we are making the sympathetic ganglion between them dysfunctional.26
Attempts have been made to establish an international nomenclature capable of standardizing surgical treatment to allow comparisons between surgical techniques and outcomes. Several anatomic landmarks have been used to determine the exact location of sympathetic interruption, including the thoracic ganglion (G), the vertebral level, and the intercostal space. More recently, a rib-oriented nomenclature has been suggested that refers to the rib level (R) instead of the vertebral level for sympathetic interruption.27 This decision was based on too many patients having mediastinal fat that can obscure clear identification of the specific ganglia and because there are many anatomic variations in the ganglion anatomy. Information about the technique used for ganglionic interruption should also be included in the nomenclature, stating whether clipping, cauterization, or segment removal is performed. For this chapter, we use the ganglionic level as anatomic reference.
Sympathetic Innervation of the Ocular Structures
The sympathetic preganglionic fibers controlling the smooth muscles of the eye are rostral, from anterior roots of G1 and G2. The fibers enter the sympathetic chain by the corresponding ganglia but do not form synapses. The synapses are subsequently formed when they ascend to the superior cervical ganglion. The postganglionic fibers, through the carotid plexus, head toward the ocular-pupillary apparatus. Consequently, resection of the stellate ganglion causes Claude Bernard–Horner syndrome (enophthalmos, myosis, and palpebral ptosis).
Sympathetic Innervation of the Cephalic Segment
Sympathetic innervation of the head and neck originates from the first to fifth thoracic medullary segments. The preganglionic fibers ascend the sympathetic chain and form synapses with the first thoracic ganglion and the inferior cervical ganglion. Most of the postganglionic fibers responsible for innervation of the face originate from G2, which implies that craniofacial sweating diminishes through G2 ablation.
Sympathetic Innervation of the Heart
Sympathetic innervation of the heart is supplied higher from the three heart nerves (superior, medium, and inferior) arising from the three cervical ganglia and also from the sixth or seventh thoracic paravertebral ganglia. Most fibers converge at the cardiac plexuses. These nerves are more abundant in the fourth and fifth thoracic segments than in the higher levels.
Physiology
The sympathetic chain supplies the smooth muscles of the blood vessels through adrenergic fibers and the sweat glands through cholinergic fibers. In the vascular system, which is different from other systems, there is no antagonistic innervation between sympathetic and parasympathetic vasoconstrictor fibers; thus, vasodilatation results from a decrease in sympathetic activity.
The autonomic nervous system has a considerable influence on vessels with greater development of the smooth muscle layer of the vessel wall in relation to its caliber. Hence, arterioles are most affected by sympathetic activity. Consequently, the autonomic nervous system has a great influence on skin circulation and is of little importance in great vessels and muscular arteries.
Eccrine sweat glands, which are responsible for hyperhidrosis, are innervated by the nonmyelinic C fibers of the sympathetic nerves, and acetylcholine is the chemical mediator. Local or systemic administration of cholinergic agents induces sweating, whereas the use of atropine blocks sweating. Although sweating in the palmar and plantar regions may result from emotional stimuli, and abundant sweating is observed under clinical conditions in which catecholamines are released by the adrenal glands, administration of adrenergic agents through any route does not produce stimulation of the sweat glands.15 Blocking of preganglionic fibers does not stop sweating caused by stimulation of the postganglionic fibers, nor does the local administration of cholinergic agents. However, if postganglionic fibers are cut, such secretion will no longer occur through local stimulation by any pharmacologic agent. This is an exception to Cannon’s law (when one unit in a series of efferent neurons is destroyed, increased irritability to chemical agents is developed in the structure that has been isolated and the effect is greater in the part that is directly denervated). Heating up of the skin may cause sweating in this situation through an unknown mechanism.
Different neural centers control the various types of sweat glands in a reflex manner. Thus, emotional sweating is controlled by a cortical center, thermal sudoresis by a hypothalamic center, gustatory sudoresis by medullary nuclei, and spinal sweating by cells of the intermediate-lateral region of the spinal cord.
The nerve centers and pathways that control emotion-induced sweating are not fully known, although it seems that they are located in the frontal lobe. Emotion-induced stimuli can increase sweating, especially in the palmar and plantar regions.
Under baseline conditions, few impulses pass to the sweat glands, and nonsensory sweating (perspiration) is always present, partly because of the activity of the glands and partly because of loss of water through the epidermis.
Indications for Cervicothoracic Sympathectomy
The current indications for cervicothoracic sympathectomy are limited to essential hyperhidrosis and selected cases of critical ischemia of the hand, complex regional pain syndrome (CRPS),28 long QT syndrome refractory to clinical treatment,29 and Raynaud’s syndrome.30
Idiopathic Hyperhidrosis
Essential or idiopathic hyperhidrosis is the production of excessive quantities of sweat beyond what is required for the organism’s thermoregulatory needs. The causal mechanism is not exactly known, but it is accepted that it is a result of stimulation of the sympathetic nervous system at the central level rather than of exercise or heat.31
Hyperhidrosis is most typically limited to the palms, soles, or axillae in a symmetrical manner. It may also affect the craniofacial segment. Hyperhidrosis may arise during childhood, but it is more intense during adolescence, a transitional period of life in which the great psychological stress associated with hormonal and sexual maturation triggers or worsens conditions that are associated with a psychosomatic component, such as hyperhidrosis. This condition may persist into adulthood, but it decreases in intensity in some patients.22 Hyperhidrosis affects approximately 3% of the population, and in 13% to 57% of patients it can be associated with a family history of hyperhidrosis.32 Climate is not an etiologic factor, but hot weather exacerbates sweating.33
Palmar hyperhidrosis generally takes on greater clinical significance than plantar or axillary hyperhidrosis because it creates significant problems within the educational, social, professional, and affective spheres that can worsen any emotional issues such patients may already have. These individuals moisten everything that they touch, which makes writing, reading, and school activities difficult. From a social and affective point of view, these patients withdraw from the world by avoiding handshakes, parties, dances, and dating.34 They tend to be almost constantly holding a cloth so they can dry their hands.35
Professionally, palmar hyperhidrosis may incapacitate such individuals from working in various activities. Thus, industrial workers with this condition who handle metals have been labeled rust-makers because of the corrosive action of their sweat.36 Other activities may become dangerous under these circumstances, such as situations in which patients handle electrical and electronic equipment.
Plantar hyperhidrosis is frequently associated with palmar or axillary hyperhidrosis and is worsened by the use of closed shoes, which hinder evaporation and favor skin maceration. The constant dampness provides conditions for fungal or bacterial infections, thereby causing a bad smell, not only on the feet but also from socks and shoes.37
Axillary hyperhidrosis tends to appear at puberty with the increased production of sexual hormones. This causes social embarrassment to patients because the sweat runs down the body and dampens and damages their clothes. These patients avoid wearing colored clothes and sometimes use rolls of paper or even sanitary pads in their axillae. Symptoms of axillary hyperhidrosis are disabling both professionally and socially for almost all patients who seek surgical treatment.38 Likewise, craniofacial hyperhidrosis and facial rubor may cause social phobia.39–41
Nonsurgical treatment may be initially attempted in all cases of hyperhidrosis. However, a constant adherence is limited because the results are usually inferior to the patient’s expectations. The available alternatives include botulinum toxin injection,42 oxybutynin,43,44 and glycopyrrolate.45
Sympathectomy is indicated for patients who do not present improvement in quality of life despite appropriate nonoperative treatment and are willing to accept the risks involved with surgical treatment (mainly compensatory hyperhidrosis).46,47
Ischemia of the Hand
Selected patients with hand ischemia, particularly those with thromboangiitis obliterans and distal arterial obstructions, ulcers in the fingers, or ischemic pain, may benefit from sympathectomy.48
Thromboangiitis obliterans, also known as Buerger’s disease, is an obliterative disease characterized by inflammatory changes in the small and medium-sized arteries and veins (see Chapter 79). The lower limbs are more frequently affected, but the upper limbs can also be compromised. Thromboangiitis obliterans usually occurs in young men (between 20 and 40 years of age) who smoke cigarettes. The cause is unknown, but it has not been documented in nonsmokers, thus implicating cigarette smoking as a primary etiologic factor.49 Occlusions start in the distal vessels of the extremities, progress proximally, and culminate in distal gangrene. Frequently, sympathetic overactivity is observed (coldness, excessive sweating, and cyanosis), probably caused by persistent and severe pain.50
Clinical treatment consists of the patient’s giving up smoking and avoiding vasoconstriction from exposure to cold or drugs together with supportive care toward controlling pain with the use of analgesics. Bypass grafts are seldom helpful because thromboangiitis obliterans rarely affects large vessels.
For selected cases of critical ischemia of the hands, cervicodorsal sympathectomy has been used to improve cutaneous vasodilatation, to control ischemic rest pain and vasomotor phenomena, and to assist in healing of the skin if patients do not respond to conservative management. However, there is no randomized comparison with other treatments, and because the disease can be improved by smoking cessation, it is difficult to judge the benefit of sympathectomy in published studies.51,52
Complex Regional Pain Syndrome
CRPS, also known as causalgia, reflex sympathetic dystrophy, posttraumatic pain syndrome, shoulder-hand syndrome, and Sudeck’s atrophy, is a term that has been used since 1994. It describes a regional pain condition that often occurs after injury, is disproportionate to the inciting event, and is associated with signs of vasomotor dysfunction and sudomotor activity. It often results in impairment of motor function.53 CRPS is categorized into two subtypes, type I (formerly reflex sympathetic dystrophy) and type II (formerly causalgia) (see Chapter 85).
When CRPS is left untreated, hyperalgesia, allodynia, signs of vasomotor dysfunction, and edema can be seen initially. After 3 to 6 months, there is increased pain, and sensory dysfunction and motor or trophic changes (or both) develop (dystrophic stage). Finally, the pain decreases, but there are still sensory disturbances (atrophic stage).54,55
Treatment includes physical therapy (indispensable), psychotherapy, pharmacologic therapy, and surgical therapy. The key to success is starting in the early stages of the disease process.56 Pharmacologic therapy includes anti-inflammatory agents, corticosteroids, anticonvulsants, antidepressants, sympatholytic drugs, and other drugs used to treat any type of neuropathic pain. Opioid analgesics are useful occasionally, but their use remains controversial.
Sympathetic blockade with local anesthetic has been used to control pain in selected patients. If it is effective, this technique can be repeated, together with physical therapy to recover functionality of the limb.57 Peridural or intrathecal infusions of anesthetic drugs can be used in selected patients who do not respond to the conservative treatment. Because of proximity to receptor sites, the therapeutic effect of intrathecal drug application lasts longer and the rate of systemic side effects is reduced. However, there are catheter-related technical problems, such as catheter dislocation, obstruction, kinking, and disconnection or rupture, as well as drug-related side effects.58
Spinal cord stimulation is efficacious in CRPS type I (level B recommendation) that is resistant to medication or other treatments. High-frequency transcutaneous electrical nerve stimulation and repetitive transcranial magnetic stimulation are noninvasive and suitable as preliminary or add-on therapies and provide satisfactory pain relief to many patients, including those resistant to medication or other therapies.59
Chemical sympathectomy with phenol or alcohol seems to have at best a temporary effect limited to cutaneous allodynia. Because studies reported in the literature have only a few patients and poorly defined outcomes, well-designed studies on the effectiveness of the procedure are needed.60,61
Sympathectomy can be used in selected patients who do not respond to nonsurgical treatment or in those who have good but transient benefit from pharmacologic sympathetic blockade (see Chapter 85).
Long QT Syndrome
Long QT syndrome, an idiopathic congenital disorder characterized by a lengthened QT interval on the electrocardiogram, is associated with a high incidence of severe tachyarrhythmia, syncope, and sudden death. The young age of most of these patients and the high morbidity and mortality in untreated individuals have led to a search for effective therapies.
There is no clinical or radiologic evidence of heart disease. Severe episodes typically occur during intense physical exercise or emotional crises, which leads to the supposition that the sympathetic nervous system plays an active part in the genesis of the problem. The mortality rate in untreated patients reaches as high as 78%. Beta blockers are effective in preventing such crises in 75% to 80% of the cases.62
Sympathectomy is only potentially indicated in patients who even with appropriate clinical treatment continue to have syncopal crises (about 20% to 25% of the patients).63
Raynaud’s Syndrome
Raynaud’s disease and phenomenon are characterized by episodic spasm of arterioles, usually in the digits, with intermittent pallor or cyanosis, and are precipitated by exposure to cold, emotional upset, or drugs. Raynaud’s disease, most common in young women, is idiopathic. Raynaud’s phenomenon is secondary to other conditions, such as connective tissue disorders, blood diseases, neurologic disorders, obstructive arterial diseases, trauma, drug intoxications (ergot), dysproteinemias, and primary pulmonary hypertension (see Chapter 122).
During the crisis, patients may complain of pain, hypothermia, numbness, and paresthesia in the affected fingers. When these episodes are frequent and intense, they may cause obstruction of arteries in the fingers and palms that subsequently results in ischemic lesions in the fingers, which are very painful and resistant to healing.
The treatment of Raynaud’s syndrome is essentially nonoperative. Sympathectomy has been used in rare patients who despite adequate clinical treatment continue to have severe symptoms or trophic lesions that heal with difficulty. However, it is difficult to judge the benefit of such sympathectomy because randomized trials have not been performed and the natural history is variable.
Surgical Technique
Open Surgery
Until the 1990s, and before VATS, open surgery was the “gold standard” for cervicodorsal sympathectomy. Several approaches are available for open surgery, each with its own advantages and disadvantages. There are three main approaches, the paravertebral, transthoracic, and supraclavicular routes. Nowadays, the open technique is indicated only when VATS cannot be accomplished because of technical reasons or an associated open operation is being performed.
The paravertebral route, mainly used by neurosurgeons, offers wide exposure of the sympathetic chain. However, it involves extensive dissection and the sectioning of several muscle bunches and requires a long period of recovery.64
The transthoracic axillary approach has the advantages of superior exposure, easier access to the sympathetic chain for wide incisions, lower risk of Horner’s syndrome, and good cosmetic results. The main complication is postsympathetic neuralgia, which lasts long and extends the recovery time.65
The supraclavicular approach requires an extrapleural access and thus allows the operation to be accomplished bilaterally in a single operation. The resulting scar becomes virtually invisible in a short time, convalescence is fast with little pain, hospital stay is short, and surgical complication rates are low. The disadvantage is that the stellate ganglion is the point of reference for identifying the sympathetic chain, and the simple manipulation can result in Horner’s syndrome, although in most cases it is transitory.66
Video-Assisted Thoracoscopic Sympathectomy
At present, VATS is considered the gold standard for cervicothoracic sympathectomy. Several approaches (one port, two ports, three ports, four ports, lateral, dorsal) are available, each with its own advantages and disadvantages.67,68 An easy and practical technique (two ports) is described in the following paragraphs.
Instrumentation
The basic equipment includes a 15-degree angled thoracoscope, video camera with monitor, DVD recorder, light source, video endoscopic instruments, electrocautery (harmonic or not), and nerve hook and vascular clips.
Anesthesia
The patient usually undergoes double-lumen endotracheal general anesthesia, which makes it possible to stop the patient’s ventilation and consequently to collapse the lung on the side that will undergo surgery. When necessary, bronchoscopy is used to verify tube positioning. A double-lumen endotracheal tube is used in patients undergoing resection of the fourth ganglion of the thoracic sympathetic chain. When thermoablation is performed on the second or third ganglion, a single-lumen tube may be used in conjunction with adequate control over lung ventilation.
Long-acting anesthetic agents are avoided to allow immediate extubation at the end of the procedure.
Positioning
The patient is placed in dorsal decubitus, semiseated position with the trunk raised approximately 45 degrees. Two small pads are placed under the shoulders to create a space between the axillae and the surgical table and to bring the shoulders forward, thereby avoiding distention of the brachial plexus when the arms are positioned in 90 degrees of abduction on the arm rests. Another pad under the knees and a securing strap at the hip level allow the legs to be positioned comfortably and impede patient movement on the surgical table when it is rotated laterally to bring the sites for surgery forward (right or left).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


