Chapter 120
Upper Extremity Arterial Disease
Revascularization
Sean P. Roddy, Philip Paty
Upper extremity arterial occlusive disease is responsible for less than 5% of all cases of limb ischemia.1 Even in high-volume centers, arm reconstructions account for only 3% of elective limb revascularizations. Palmar and digital artery occlusive disease is the most common cause of upper extremity ischemia, whereas large-vessel disease, including arteries proximal to the wrist, accounts for less than 10% of cases of upper extremity arterial occlusive disease. The majority of arm emboli are cardiac in origin (75%). The most common site for emboli is the brachial artery (60%), followed by the axillary artery (26%). In situ thrombosis accounts for only 5% of episodes of arm ischemia.2 Usually there is an underlying embolic source such as atrial fibrillation. This chapter deals with revascularization for both acute and chronic ischemia involving the intrinsic arteries of the upper extremities, including the axillary, brachial, radial, ulnar, and palmar arteries. Raynaud’s syndrome (see Chapter 122), thoracic outlet obstruction (see Chapter 127), occlusive disease of the great vessels (see Chapter 105), Takayasu’s Arteritis (see Chapters 79 and 80) are described elsewhere. Because the volume of these procedures is small, it is difficult to draw evidence-based conclusions on the optimal approach for patients with upper extremity ischemia. What is known is that patients with diabetes mellitus and chronic renal failure make up a significant proportion of those with digital gangrene. Treatment presents a challenge that often involves difficult reconstruction, meticulous wound care, optimal medical management of the contributing disease process, and patience.
Clinical Findings
Patients may have either acute or chronic ischemic symptoms, as discussed in detail in Chapter 119.
Acute Arm Ischemia
Arms with acute ischemia account for only one fifth of all acutely threatened limbs. Women are affected twice as often as men, and patients are significantly older than those with acute symptoms in the lower extremity.3 Because most reports of acute arm ischemia generally involve those who required surgery, the denominator including those managed medically is difficult to calculate. The classic symptoms involve the “six P’s”: pain, pallor, paresthesia, paralysis, pulselessness, poikilothermia. The fingers and hand are often cool with obvious color changes. Numbness and tingling are common. Motor dysfunction may range from mildly diminished hand strength to paralysis. Wrist pulses are usually absent while axillary and/or brachial pulses may remain palpable. Doppler interrogation at these sites often produces an occlusive or “water hammer” signal. General principles grading ischemic clinical findings and the urgency to reestablish perfusion to the upper extremity should be considered similar to those described for acute lower limb ischemia (see Chapters 108 and 109).
Chronic Arm Ischemia
Whereas chronic leg ischemia is usually due to atherosclerosis, the causes of chronic arm ischemia are more diverse. Although atherosclerosis is still one etiology, consideration must be given to other pathologies such as thoracic outlet syndrome, iatrogenic injury after arm catheterization, immediate or delayed traumatic injury, rheumatoid arthritis, collagen vascular disease, and rarer causes such as Takayasu’s or giant cell arteritis and radiation-induced injury. Hand and finger ischemia has been reported with increasing frequency in patients with end-stage renal disease.4,5 The etiologic backgrounds of this disease process are multiple and include thrombosis, accelerated atherosclerosis, and diffuse arterial calcification. The origin of this condition may well involve atherosclerosis, but there is a well-described association with calciphylaxis.6 Manifestations range from digital pain at rest and ulceration to gangrene. Initial assessment is by noninvasive studies such as pulse volume recordings or duplex ultrasound, followed by magnetic resonance or computed tomographic angiography. Confirmation of these findings can be obtained by aortic arch and arm catheter-directed angiography. Occlusive lesions will be located in the axillary, brachial, forearm, or palmar/digital arteries. Each requires separate treatment strategies.
Another clinical situation of hand ischemia encountered in patients with end-stage renal disease is that associated with an ipsilateral upper extremity arteriovenous fistula. The onset of hand ischemia is a devastating complication of upper extremity hemodialysis access. Arteriovenous shunts are almost always associated with some degree of reduced arterial flow to the distal circulation. Untreated, this may produce pain, ulceration, and gangrene in a previously viable extremity. Although prevention of this complication remains paramount, several techniques are available to manage this problem once diagnosed. Chapter 77 describes the diagnosis and management of steal-associated ischemia and critical upper extremity ischemia caused by infrabrachial disease in renal failure patients.
The decision to intervene in those with chronic arm and hand ischemia requires understanding of the underlying pathology and likelihood of success with potential revascularization options. Unlike the lower extremity, the natural history of effort-induced arm pain (a claudication equivalent) and ischemic hand rest pain is not well documented. This said, it would seem analogous that critical limb ischemia should undergo arterial reconstruction, whereas arm “claudication” should be treated selectively. Aspects of preoperative preparation and perioperative care, as delineated in Section 6, Chapters 31-33, apply.
Treatment
Conservative Therapy
Between 9% and 30% of patients seen by vascular surgeons with upper extremity arterial occlusive disease are managed nonoperatively because of significant co-morbid conditions or minimal symptomatology. Postoperative mortality rates for brachial embolectomy are as high as 12%.7 However, after successful brachial embolectomy, 95% of patients will remain free of symptoms.8 Patients managed conservatively are probably underreported in the literature. In the few reported series, assessment of symptoms and disability tends to be inconsistent. However, in a series of 95 patients described by Baird and Lajos in 1964 with arm ischemia managed without surgery, 32% were left with permanent disability in the arm.9 In 1977, Savelyev and coauthors reported that 75% of patients managed conservatively had a poor functional outcome.10 More recently, in 1985 Galbraith and associates confirmed that 50% of their nonoperatively managed patients had persistent exercise-induced forearm pain (a claudication equivalent).11 Therefore, although conservative management is appropriate for some patients with acute ischemia, for those with a reasonable life expectancy, all efforts should be made to restore blood flow.
Endovascular Treatment
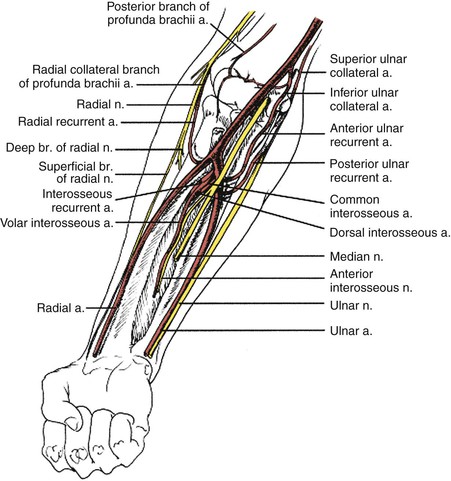
Endovascular therapies for the treatment of arterial occlusive disease have increased in popularity over the last decade with the improvement in catheter, balloon, and stent technology. The majority of lower extremity revascularizations in large vascular surgery practices are now done percutaneously. However, the upper extremity has not shared the same paradigm shift, possibly because of the infrequency of interventions or the causes of the arterial disease. Most institutional reviews that describe treatment of occlusions in the axillary, brachial, radial, and ulnar arteries still involve surgical bypass or embolectomy.12,13 Use of covered stents in axillary injury is becoming more frequent. There are now many reported cases that indicate this technology is feasible with reasonable early results; however, long-term durability remains to be determined.14 An example of such therapy is illustrated in Figure 120-1. There are also reports of small series of patients treated with axillary artery angioplasty for radiation-induced occlusion, by brachial artery atherectomy, and by radial artery stenting for digital gangrene, but their numbers are low and follow-up is minimal.15–18 Therefore, because open reconstruction remains the mainstay of treatment, dissection and exposure of the arterial anatomy for open revascularization will be described at the most common levels of intervention (Fig. 120-2).
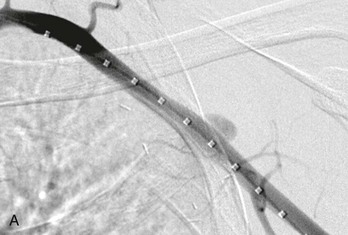
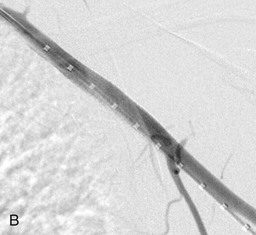
Figure 120-1 A, Angiography of the left arm after penetrating trauma reveals a pseudoaneurysm off the axillary artery. B, After deployment of a covered stent graft in the axillary artery, no further extravasation is seen.
Surgical Treatment
Arterial Exposure
Axillary Artery.
The patient is placed supine and the arm is draped circumferentially. The axillary artery is exposed with a transverse incision 2 cm below the middle third of the clavicle. The underlying pectoralis major muscle is divided, when possible, in the decussation between the sternal and clavicular portions. Despite the descriptions found in most operative texts, this point is not always readily apparent. Division of the pectoralis major exposes the clavipectoral fascia, which is divided. The axillary artery is located cephalic to the vein. It is dissected carefully to avoid injury to the surrounding cords of the brachial plexus. The second part of the axillary artery can be exposed by dividing the pectoralis minor muscle (Fig. 120-3A). If needed, the distal third of the axillary artery may also be exposed. An oblique incision is made along the lateral margin of the pectoralis major muscle with the arm abducted 90 degrees relative to the thorax. Once the subcutaneous tissue is divided, the axillary sheath is located near the posterior and inferior border of the coracobrachialis. Careful dissection avoids injury to the medial and lateral cords of the brachial plexus medially and the median and ulnar nerves laterally (Fig. 120-3B).
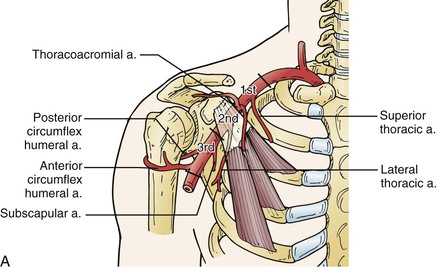
Figure 120-3 Exposure of the axillary artery. A, Segments of the axillary artery are defined by relationship to the coracoid process and the pectoralis minor muscle. This muscle can be divided to provide full axillary artery exposure. B, The brachial plexus is intimately associated with the axillary artery at all levels and must be avoided during dissection.
Brachial Artery.
The proximal or mid brachial artery is exposed through a medial incision over the bicipital groove. This allows access to the proximal or middle third of the brachial artery. The basilic vein and cutaneous branches of the median nerve are located within the subcutaneous tissue and should be avoided during dissection. Traction or transection of the median antebrachial cutaneous nerve may lead to hyperesthesia or anesthesia along the medial dorsal surface of the forearm. This occasionally occurs after dialysis access (e.g., basilic vein transposition) and can be debilitating for the patient. In some cases it can even preclude use of a patent and mature fistula. The brachial sheath is then incised longitudinally. The median nerve is the most superficial structure encountered. The nerve is gently mobilized and retracted to allow access to the brachial artery. Crossing vein branches should be divided to minimize the risk of injury to the posteriorly located ulnar nerve.
The distal third of the brachial artery and its bifurcation, in contrast, are exposed in the antecubital fossa (Fig. 120-4). A lazy S–shaped incision is recommended to expose the origins of the ulnar and radial arteries. Sometimes a vertical incision is used. However, there is a legitimate concern about contracture of the elbow with vertical incisions. The skin and subcutaneous tissues are divided. Care is taken to preserve the superficial veins, especially the median antecubital vein, because it may be required for autogenous patch angioplasty closure. The bicipital fascia is incised and the brachial artery is seen coursing between the biceps tendon laterally and the median nerve medially. Dissection is continued distally until the ulnar and radial arteries are encountered. The radial artery is really a continuation of the brachial artery. The ulnar artery, on the other hand, comes off the brachial artery medially and, within 2 to 3 cm, dives beneath the pronator and epitrochlear muscles. In 20% to 25% of individuals, the brachial artery may have anatomic variation that typically involves a high bifurcating system.
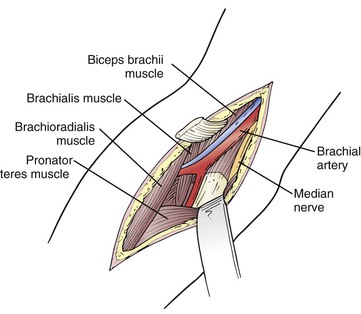
Figure 120-4 Exposure of the brachial artery at the elbow. The bifurcation of the brachial artery can be exposed by retracting the pronator teres and flexor muscle mass. The radial artery can be followed the length of the incision, but the larger ulnar artery dives between the heads of the flexor digitorum superficialis.
Radial Artery.
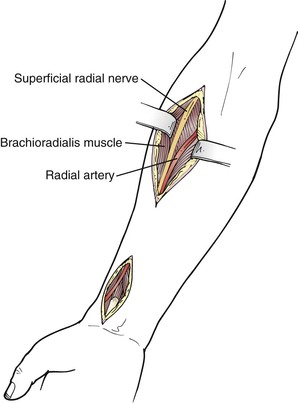
The course of the radial artery in the forearm follows an oblique line from the brachial artery pulse medial to the biceps tendon to the styloid process of the radius. In the midforearm, the radial artery is medial to the brachioradialis and lateral to the flexor carpi radialis muscles. A lateral longitudinal incision is made. The muscles are separated to reveal the radial artery as needed (Fig. 120-5).
At the wrist the radial artery is exposed by a longitudinal incision between the tendons of the flexor carpi radialis and brachioradialis muscles. This is the traditional site of the radial artery pulse in normal subjects. The artery is superficial and exposure is relatively straightforward. The superficial branch of the radial nerve is often located near the lateral aspect of the artery. Injury can result in troublesome paresthesias along the lateral aspect of the thumb.
Ulnar Artery.
The ulnar artery extends from the medial epicondyle of the humerus to the pisiform bone. In the midforearm, the ulnar artery lies beneath the deep fascia between the bellies of the flexor digitorum laterally and the flexor carpi ulnaris medially (Fig. 120-6). The ulnar nerve joins the artery on its lateral aspect for its distal two thirds. It may be injured if not carefully identified and preserved.

Figure 120-6 Exposure of the forearm ulnar artery. The ulnar artery in the midforearm is reached between the flexor carpi ulnaris and flexor digitorum superficialis.
At the wrist, the ulnar artery is lateral to the flexor carpi ulnaris (it is the most medial tendon palpable at the wrist). For exposure, this tendon is identified and a vertical skin incision is made lateral to it. The ulnar artery is relatively deeper than the radial artery at the wrist but just as easily exposed. The palmar cutaneous branches of the ulnar nerve are superficial to the artery here and should be preserved.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree