38 Upper Extremities and Aortic Arch
 Introduction
Introduction
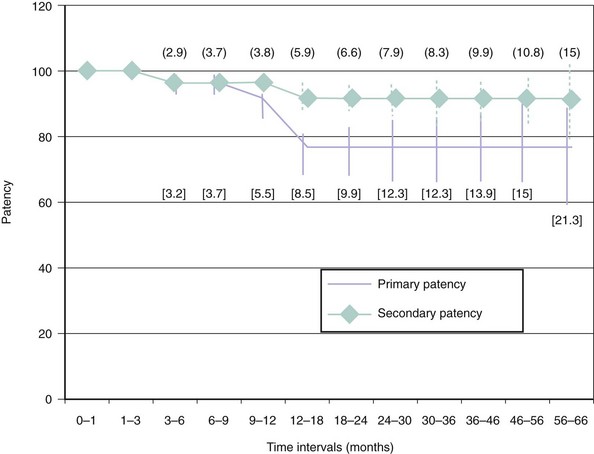
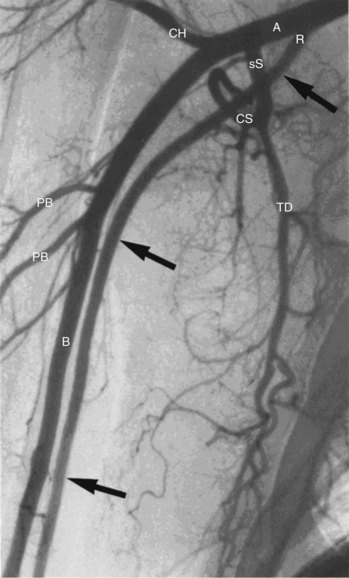
In the past decade we have seen the scope of interventional cardiology widen to encompass nearly the whole realm of vascular medicine, including arterial diseases of the proximal arch and upper extremity. The extension of cardiology to peripheral artery disease (PAD) has been a natural outgrowth for several reasons. First, the major risk factors for PAD are identical to those for coronary artery disease (CAD); hence there is considerable overlap in the affected patient populations. There is a high degree of correlation between the degree of atherosclerosis in the brachial, carotid, and coronary arteries.1 The cardiologist will, therefore, invariably have initial exposure to these patients when they present for healthcare. Second, patients with PAD are at extremely high risk for morbidity and mortality from myocardial infarction (MI) and stroke and should arguably be regularly followed by a cardiologist for medical care.2–5 Indeed, the majority of patients with PAD do not require surgical or catheter-based intervention and will derive their greatest benefits in terms of decreased morbidity and mortality simply from risk-factor reduction and best medical therapy. Third, the tools and techniques that work for intervening in atherosclerosis of the coronary arteries are translatable to the large arteries of the peripheral vasculature. In most cases the same standard catheter skills that are used for coronary cases can be applied to noncoronary arch and upper extremity cases.6 There is high primary success in treating proximal arch and upper extremity occlusive disease as well as reasonable long-term patency (Fig. 38-1). In some cases catheter-based therapies have become superior to traditional surgical techniques.7 It has been estimated that atherosclerosis of the upper limbs accounts for 5% of PAD affecting the extremities.8,9 Even so, when one considers that 25% to 40% of the patients seen in a typical cardiology clinic have significant PAD,2,3,5 it becomes apparent that proximal arch and upper extremity arterial diseases constitute a significant disease burden. The PARTNERS trial revealed that PAD is underdiagnosed and undertreated2; this is especially true for arterial diseases of the proximal arch and upper extremities. Unlike PAD of the lower extremities, which is almost exclusively caused by atherosclerosis, the differential diagnosis for arterial disease of the proximal arch and upper extremities is extensive. While the relative infrequency of significant atherosclerosis in the proximal arch vessels and upper extremities (as opposed to the lower extremities) probably represents underlying differences in pathophysiology (e.g., turbulence, inflammation, oxidative stress, density of receptors), its low prevalence is likely also a result of our collective failure to diagnose it. The comparatively low prevalence of symptomatic arterial disease affecting the proximal arch and upper extremities combined with the complex differential diagnosis underlying these diseases make timely and accurate diagnosis problematic. Many of these patients, such as those with subclavian steal (coronary or vertebral artery) or thoracic outlet syndrome, are misdiagnosed and/or inappropriately treated. This is unfortunate because, when correctly diagnosed, these patients are highly treatable, and those with atherosclerotic PAD stand to benefit greatly in terms of decreased rates of cardiovascular events by receiving the appropriate medical therapy.
 Objectives
Objectives
 Normal Anatomy
Normal Anatomy
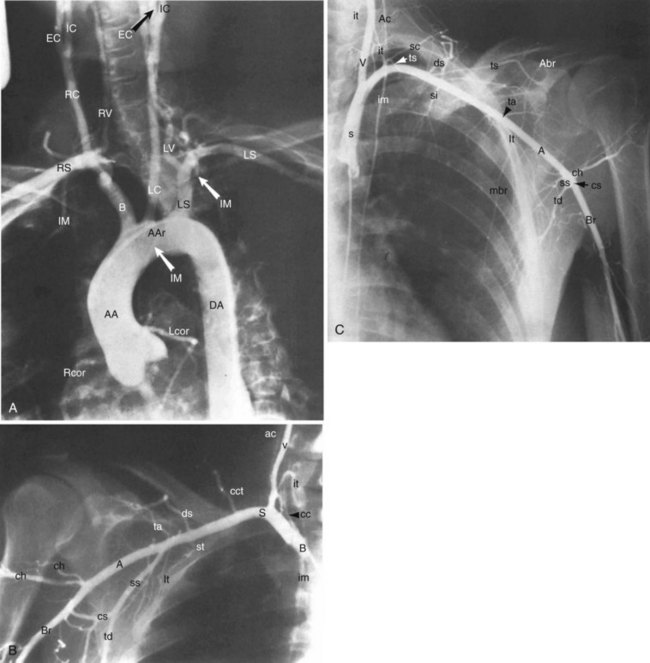
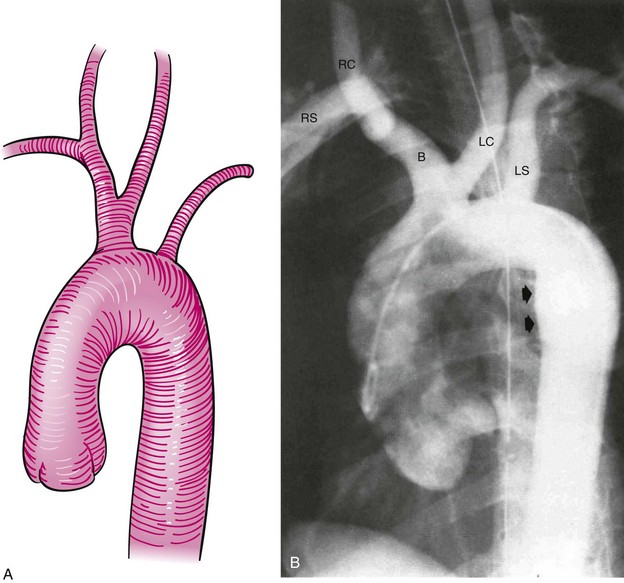
The arterial system of the upper extremities (Fig. 38-2) is analogous to that of the lower extremities. In 70% of the population,10 the arteries of the proximal arch are the brachiocephalic (innominate) artery on the right (which quickly divides into the right subclavian artery and the right common carotid artery), and the left common carotid artery and the left subclavian artery, which come off the arch separately, on the left. The subclavian artery becomes the axillary artery at the lateral border of the first rib. The axillary artery becomes the brachial artery, the first intrinsic artery of the upper extremity, in the shoulder girdle at the border of the teres major muscle. The brachial artery is superficial and should be palpable throughout its course. It first lies medial to the humerus and then courses anterior to it. Just below the antecubital fossa, the brachial artery divides into the ulnar artery, which courses medially, and the radial artery, which courses laterally. The ulnar and radial arteries are also superficial and normally palpable. In the wrist, the distal ulnar artery forms the superficial palmar arch and the distal radial artery forms the deep palmar arch. The palmar arches form extensive anastomoses before giving off the true digital arteries (medial and lateral).11 There are extensive collaterals formed by lesser arteries around the shoulders and elbows and in the palms. These collaterals form a parallel arterial circuit that allows normal perfusion in the upper extremities even when there is a significant occlusion—with one major exception. In contrast to most other large vascular beds, each upper extremity is supplied by only one artery coming off of the aorta. Therefore all anastomoses in the extremities are distal to the aortic branch point, resulting in the potential for ischemic vulnerability and—as discussed further on in considering the upper extremities—for various steal phenomena.
 Anatomical Variants
Anatomical Variants
The presence of anomalous circulation in the arteries of the proximal arch and upper extremity (cumulatively in about 30% of the population)10,12 can lead to misdiagnosis and confusion in the catheterization lab. Here we review some of the most prevalent anatomical variants. (Anatomical variants associated with thoracic outlet syndrome are described separately later in the chapter.)
Bovine Arch
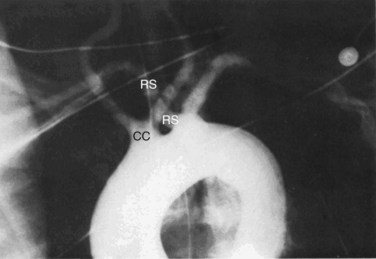
The bovine arch is the most common anatomical variant of the aortic arch, occurring in 22% of the population and accounting for 73% of all arch vessel anomalies.10,12 It consists of a common origin of the right brachiocephalic and left common carotid arteries (Fig. 38-3). It is not associated with any pathological significance.
Left Vertebral Artery Originating from the Aorta
Normally the left vertebral artery comes off the left subclavian artery. In 4% to 6% of the population it originates directly from the aortic arch and accounts for 14% of arch vessel anomalies.10,12 It is not associated with other anomalies and does not confer any pathological predilection, although it may predispose to more upper extremity symptoms in the presence of proximal left subclavian artery stenosis owing to the absence of a significant collateral pathway.
Aberrant Right Subclavian Artery
Aberrant right subclavian artery (ARSA, or arteria lusoria) is an example of a vascular ring with pathological significance (Fig. 38-4).13–15 It is caused by the persistence of the posterior segment of the fourth aortic arch. It manifests as a right subclavian artery arising from the left aortic arch distal to the origin of the left subclavian artery and follows a retroesophageal course. It occurs in less than 2% of the population and is associated with other anomalies of the arch vessels. The proximal section of an ARSA has a propensity for aneurysm (termed Kommerell’s diverticulum) and may cause symptoms related to compression of nearby structures. The most common symptom is intermittent dysphagia (dysphagia lusoria) due to compression of the esophagus.16 However, regardless of symptoms, the presence of Kommerell’s diverticulum is an indication for surgical repair because of its significant risk of rupture.17,18 Interestingly, surgical repair of ARSA by way of ligation of the anomalous origin has been described as an iatrogenic cause of the subclavian steal syndrome.18
Aberrant Left Subclavian Artery
An isolated left subclavian artery arises from a right aortic arch when there is interruption of the left fourth arch proximal to the seventh cervical intersegmental artery. This anomaly is a rare cause of upper extremity ischemia and has been associated with subclavian steal syndrome (Fig. 38-5).10,19 As above, its repair has also been associated with the development of the subclavian steal syndrome.18
Brachial, Radial, and Ulnar Arteries
In 20% of the population, the brachial artery is doubled during all or part of its course. Owing to extensive collateralization around the elbow, occlusion of one of these branches is generally well tolerated. In 15% to 20% of the population, the radial artery originates early on from the brachial artery or even as high as the axillary artery (Fig. 38-6).10 It is relatively uncommon, however, for the ulnar artery to branch off prematurely (prevalence 1%-3%). The ulnar artery is congenitally absent in 2% to 3% of the population. These variants confer no intrinsic pathological significance.
 Classifications
Classifications
Given the broad differential diagnoses that one must consider in approaching suspected arterial disease of the proximal arch vessel or upper extremity, it is useful to classify these diseases based on symptom acuity (time of onset) and size of the involved artery. The diseases involving large or medium-sized arteries, such as those proximal to the wrist, are most relevant to the cardiologist and are caused by one of five mechanisms (Table 38-1). The small-vessel diseases (i.e., those occurring distal to the wrist) are frequently rheumatological or hematological disorders and include blood dyscrasias, Buerger’s disease, Henoch-Schönlein purpura, the various hypercoaguable states, and multiple small-vessel vasculitides. It can be clinically difficult to distinguish between large- and small-vessel disease. For example, although most think of Raynaud’s phenomenon as a small-artery disease, it is a common feature of both large- and small-vessel disease.
TABLE 38-1 Diseases of the Large- and Medium-Size Arteries
| Site/Disease State | |
|---|---|
| Atherosclerosis | Brachiocephalic artery |
| Subclavian artery | |
| Axillary artery | |
| Aneurysm | Thoracic outlet syndrome |
| Trauma, crutch syndrome | |
| Vasculitis | |
| Fibromuscular dysplasia | |
| Thromboembolism | Cardiac |
| Aortic arch | |
| Proximal great vessels | |
| Entrapment | Thoracic outlet syndrome |
| Trauma | |
| Neoplasm | |
| Vasculitis | Giant cell (temporal) arteritis |
| Kawasaki’s arteritis | |
| Takayasu’s arteritis | |
| Radiation-induced arteritis |
Adapted with permission from Rajagopalan S, Mukherjee D, Mohler E. Manual of Vascular Diseases. Baltimore: Lippincott Williams & Wilkins: 2004:464.
 Initial Evaluation
Initial Evaluation
History
In terms of distinguishing the vasculitides, age is often the best initial discriminator. Kawasaki’s disease is strictly a pediatric disease in onset; however, its sequelae, such as coronary and peripheral artery aneurysmal disease, can be significant in the adult population. Takayasu’s arteritis generally occurs in patients below 40 years of age, whereas giant cell (temporal) arteritis is unlikely to affect patients below 65 years of age. Thoracic outlet syndrome (TOS) is, perhaps, most difficult to tease out from history alone. Its presence is suggested by an exacerbation of symptoms (e.g., pain or parasthesia) in the context of upper extremity movement, particularly abduction. Unfortunately this feature hardly distinguishes it from the upper extremity claudication that is characteristic of subclavian or axillary artery stenosis. Except in cases of trauma, symptom onset is usually vague and slowly progressive. During the initial evaluation, the clinician should inquire about timing of symptoms, symmetry, use of potentially vasospastic medications (Table 38-2), use of recreational drugs (including tobacco), prior external beam radiation (as in the treatment of Hodgkin’s disease, breast cancer, or lung cancer), and occupational exposures (Table 38-3). It is essential to know these details before prescribing further testing. The utility of gathering a comprehensive history from the patient with suspected proximal arch or upper extremity disease cannot be overstated. It is easy to be led astray by the large differential diagnosis that must be considered. Common sense dictates that common diseases occur commonly, and this line of thinking should be applied in approaching such patients. Nevertheless, one should be prepared to hunt for the occasional zebra. For instance, in the context of upper extremity symptoms, dysphagia may suggest a rheumatologic disorder, such as CREST (Calcinosis, Raynaud’s syndrome, Esophageal dysmotility, Sclerodactyly, Telangiectasia) syndrome, or an anatomical variant, such as ARSA (Aberrant right subclavian artery) with dysphagia lusoria.
TABLE 38-2 Medication-Induced Vasospasm
| Class | Example(s) | Common Uses |
|---|---|---|
| 5-HT agonists | Sumatriptan | Migraine headache |
| Ergot alkaloids | Methysergide Ergonovine | Migraine headache Post-partum bleeding |
| Dopamine agonists | Bromocriptine | Parkinson’s disease Acromegaly Hyperprolactinemia |
| Immunomodulators | α-Interferon (α-IFN) | Hepatitis C virus Hepatitis B virus Hematological malignancies |
| Antiseizures | Phenytoin Carbamazepine | Seizure treatment and prevention |
| Antibiotics | Trimethoprim/sulfamethoxazole Tetracycline, doxycycline | Antimicrobial |
| Xanthine oxidase inhibitors | Allopurinol | Gout prophylaxis Tumor lysis syndrome Nephrolithiasis prophylaxis (calcium oxalate) |
| Diuretics (thiazides) | Hydrochlorothiazide Chlorothiazide Chlorthalidone Metolazone | Congestive heart failure Edema Hypertension |
| Aldosterone receptor antagonists | Spironolactone | Congestive heart failure Edema Hypertension (systemic) Portal hypertension Hyperaldosteronism |
| Recreational | Nicotine Cocaine | Recreational |
TABLE 38-3 Occupational Exposures Associated with Proximal Arch and Upper Extremity Disease
| Exposure | Disease State |
|---|---|
| Vinyl chloride | Raynaud’s phenomenon |
| Vibratory tools | Raynaud’s phenomenon |
| Hypothenar hammer syndrome | |
| Repetitive palmar pressure/trauma | Raynaud’s phenomenon |
| Hypothenar hammer syndrome | |
| Sporting activities | TOS |
| Hypothenar hammer syndrome | |
| Radiation | Atherosclerosis |
| Vasculitis |
 Physical Examination
Physical Examination
Like a thorough history, careful attention to the physical exam will usually narrow the differential diagnosis and reveal the best next step in the patient’s workup. For example, the absence of the brachial and radial pulses in a young Asian woman would be immediately suggestive of Takayasu’s arteritis. In the case of patients in whom proximal arch and upper extremity disease is already suspected, it is prudent to conduct a thorough (rather than focused) physical exam because the underlying pathology is so frequently a systemic process. But given the high prevalence of PAD, it is reasonable to at least perform office-based screening for upper extremity disease on all patients who present to the cardiology clinic by measuring the pulses and blood pressures in both arms. The physician should do this manually. A systolic gradient of more than 15 to 20 mm Hg is suspicious for significant proximal arch or upper extremity stenosis (although there may be no differential in patients with bilateral disease). The specificity of 10 and 20 mm Hg differentials in cuff pressures between arms is 85% and 94%, respectively.20 The brachial, radial, and ulnar pulses should be palpable and symmetrical throughout their course. Multiple pulse deficits in a young patient (as in the example above) are suggestive of Takayasu’s arteritis. TOS maneuvers, which typically entail palpation of the radial artery before and after a maneuver (usually involving arm abduction), are specifically discussed in the section on TOS (below). Allen’s test and capillary refill (<5 seconds) should be compared in each hand. Bilateral findings indicate a systemic disease process. The carotid arteries and supraclavicular fossae should be palpated and ausculated. A supraclavicular pulsatile mass raises the possibility of a subclavian artery aneurysm or associated cervical rib. Fortunately the signs of acute and chronic arterial insufficiency are usually distinct. Signs of acute arterial insufficiency are neatly summarized by the five P’s (pulselessness, pallor, poikilothermia, pain, parasthesia). Signs of chronic arterial insufficiency include muscle atrophy, skin bronzing, focal hair loss, and digital gangrene. Nonhealing skin ulcers are an exception to this rule, as they can result from either acute or chronic arterial insufficiency. The presence of skin ulceration should prompt consideration of atherosclerotic disease, embolic disease, Buerger’s disease, or vasculitis. Patients with vasculitis or malignancy often look chronically ill and may present with fever, unintentional weight loss, or frank cachexia. The presence of synovitis, nail pitting, prominent nail-bed capillary loops, and skin lesions such as erythema nodosum, pyoderma gangrenosum, petechial rash, or palpable purpura are highly suggestive of a vasculitis or other rheumatological disease. However, the constellation of fever, rash, petechiae, and nail findings also warrants the consideration of infective endocarditis with embolization to the upper extremities. Splinter hemorrhages under the nail beds on the involved side indicate an atheroembolic process, such as one originating from the heart or the ipsilateral subclavian/axillary arteries, or from infective endocarditis.
Laboratory
A few initial laboratory tests—such as a complete metabolic profile (CMP), a complete blood count (CBC), coagulation parameters, an erythrocyte sedimentation rate (ESR), and a lipid profile—are useful in narrowing the differential diagnosis. Table 38-4 gives a comprehensive list of laboratory tests organized according to a system-based differential diagnosis.
| Cardiovascular | Rheumatological |
|---|---|
| Atherosclerotic | CBC with differential |
| BMP | Rheumatoid factor |
| Lipid profile | Antinuclear antibody |
| LP(a) | Anti-dsDNA antibodies |
| hsCRP | Extractable nuclear antigens |
| HgbA1c | ESR |
| Cardiac emboli | CRP |
| aPTT | Hepatitis virus screening |
| PT/INR | Cryoglobulins |
| Cholesterol emboli | |
| CBC with differential | Hematological |
| Urine eosinophils | Malignancy |
| CMP | |
| Infectious | CBC with differential |
| CBC with differential | SPEP with IFA |
| ESR | Serum free light chains |
| CRP | Hypercoagulability |
| Blood cultures | CBC |
| aPTT, PT/INR | |
| Neurological | Factor V Leiden |
| Vitamin B12 | Prothrombin 20210 gene mutation |
| RPR | Antithrombin III |
| TSH | Antiphospholipid antibodies |
| HgbA1c | Protein C |
| hsCRP | Protein S |
| Lupus anticoagulant |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree