Other Obstructive Disorders |
INTRODUCTION
The upper airway is conventionally described as being made up of all the structures that conduct air between the carina and the nares and includes the trachea, larynx, pharynx, nasal airway, and oral airway. Upper airway structures may change their physiologic function in response to pressures around them, and anatomic structures near them. Thus, physiologically, the segments of the upper airway behave differently when they are subject to pleural pressures (anatomically intrathoracic) or ambient pressures (anatomically extrathoracic). Furthermore we now know that extrathoracic airway function may change with posture, sleep/wake state, and the function and anatomy of tissues surrounding the airway.
The upper airway evolved anatomically in humans to subserve several important functions including swallowing, breathing, and vocalization.1 The multifunctionality of the upper airway increases the risk of certain diseases (e.g., aspiration and sleep apnea).2 These functions require that different segments of the airway have differing properties. The trachea receives some support by the tracheal rings, the nasal airway is surrounded by rigid structures, and the oral airway has a rigid boney roof, the hard palate. On the other hand, the pharyngeal airway does not have rigid structures supporting it. It is a collapsible tube whose patency is maintained by muscles whose function is affected by arousal state (sleep/wake, and more specifically during sleep, the stage of sleep), the structures around it, and posture. Thus, the pharyngeal airway is divided anatomically and physiologically into the nasopharynx, retropalatal oropharynx, retroglossal oropharynx, and hypopharynx.
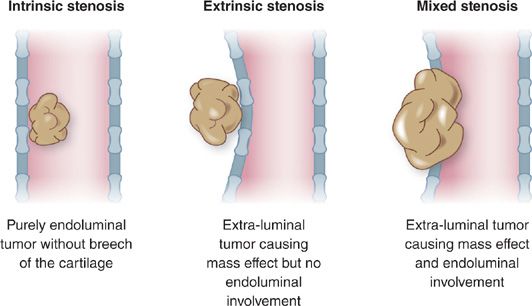
Clinically significant obstruction in adults may occur anywhere within the upper airway. Common etiologies of upper airway obstruction (UAO) include neoplasia, scar formation, skeletal facial malformations, infection, inflammatory disorders, trauma, extrinsic compression related to pathology of adjacent structures, and functional changes related to posture and sleep/wake state. Airway obstruction may be classified as extrinsic, intrinsic, or mixed (Fig. 49-1).
Figure 49-1 Classification of airway tumor involvement. A. Intrinsic stenosis. Purely endoluminal tumor without breech of the cartilage. B. Extrinsic stenosis. Extraluminal tumor causing mass effect but no endoluminal involvement. C. Mixed stenosis. Extraluminal tumor causing mass effect and endoluminal involvement.
UAO can be acute and life-threatening, or chronic and resulting in significant symptoms, some of which may not even relate to the respiratory system. Initial management of acute UAO focuses on securing the airway and stabilizing the patient. Often the intervention is surgical. Some diseases require bypassing the obstruction using translaryngeal intubation or tracheostomy. Definitive long-term management depends on the underlying etiology and physiology, and may include both medical and surgical interventions. The still evolving fields of imaging and interventional pulmonology offer new diagnostic and management modalities. This chapter provides an overview of acute and chronic UAO in adults and focuses on clinical presentation, assessment, etiology, and management. Obstructive sleep apnea is covered in Chapter 99 of this volume.
HISTORICAL PERSPECTIVE
Acute UAO can be so distressing to the patient and to those around him/her that it is not surprising that tracheostomy has been used as a treatment for at least 3000 years, as described in ancient Egyptian tablets and Greek and Roman writings.3 Tracheostomy was considered dangerous because it might lead to catastrophes (e.g., laceration of the carotid artery) or infections that may not be controlled. Even Hippocrates warned about the dangers of tracheostomy. By the mid-16th century, the tracheostomy had been performed to relieve UAO caused by a pharyngeal abscess.
Although the procedure was already widely known within the medical profession in the 18th century, it was seen as potentially very dangerous. The first president of the United States, George Washington, died of acute UAO likely caused by epiglottis while his doctors (who were familiar with tracheostomy) treated him with blood letting.4 During the 19th century, the procedure was used to treat UAO caused by croup and diphtheria.
By the early 20th century, nonsurgical treatments were used to treat UAO; for example, rigid bronchoscopy was used to remove foreign bodies from the trachea.5 The flexible fiberoptic bronchoscope was introduced in 1966, and the next four decades saw the introduction of many diagnostic and therapeutic modalities including: Nd:YAG laser, video bronchoscopy, airway stenting, endobronchial ultrasonography, endobronchial electrocautery, cryotherapy, argon laser coagulation, thermal laser therapy, photodynamic therapy (PDT), brachytherapy, and percutaneous tracheostomy.6
There are newly described causes of UAO and novel imaging techniques, both radiographic and endoscopic, which are in evolution to detect and quantify UAO. Treatment strategies are continuously advancing. The incidence of malignancy and related obstruction of the upper airway has increased due in part to tobacco use and exposure to modern environmental toxins. It is estimated that 20% to 30% of lung cancer patients present with symptomatic airway obstruction. The exact prevalence, however, is unknown as a result of the nihilistic view of lung cancer, particularly in the setting of advanced disease. Complications of endotracheal intubation and tracheostomy have become well-recognized causes of benign upper airway stenosis and malacia. Again, it is likely that this pathology is underreported and many people are inappropriately treated for asthma or other chronic peripheral airway diseases. Improvement in pharmacologic agents to treat infectious, inflammatory, and malignant etiologies, as well as developments in radiation oncology, have influenced the management of UAO. More recently, advances involving anesthetic agents and anesthesia techniques, along with development of sophisticated surgical procedures for reconstruction of the larynx, trachea, and bronchi, have had a considerable impact on the management of this condition. Development of new endoscopic and imaging techniques and introduction of interventional pulmonology also have proved useful in the management of UAO.
In the mid-1960s it became apparent that UAO occurred in some patients only during sleep and until the mid-1980s the only effective treatment was tracheostomy, which was used to bypass the segment of the airway obstructing during sleep.7 Up to that era, the main focus of the laboratory diagnosis of pulmonary disease was for patients with obstructive intrathoracic disease and soon the importance of being able to diagnose UAO became apparent.8 Soon the notion of using positive airway pressure to overcome UAO was established.9
CLINICAL FEATURES
Upper and lower airway obstruction may present with similar symptoms (e.g., shortness of breath, noisy breathing) and physical findings (e.g., wheezing, diminished breath sounds). Asthma and chronic obstructive pulmonary disease, common causes of lower airway obstruction, are often incorrectly assumed to be causing the patient’s symptoms.
Significant UAO may be asymptomatic for a prolonged period of time, resulting in delayed presentation, diagnosis and possibly a catastrophic outcome. When UAO develops or worsens acutely, asphyxia and death may result within minutes to hours. When UAO develops slowly, diagnoses may be delayed or incorrect, and, in the case of malignancy in the upper airway, may lead to incurable disease.
Dyspnea and noisy breathing, the most common symptoms of UAO, are often prominent during exercise and also may be intensified or relieved by a change in body position. The patient may indicate that breathing is labored while supine and may experience sleep disordered breathing in the forms of obstructive sleep apnea or upper airway resistance syndrome (see Chapter 99). Therefore, daytime somnolence may be a prominent feature of UAO. In severely affected patients, peripheral edema as a result of cor pulmonale may be present as a consequence of chronic hypoxemia and hypercarbia during sleep and wakefulness.
In most cases, significant anatomic obstruction precedes the development of symptoms. By the time dyspnea on exertion occurs, the airway diameter at the site of obstruction is likely to be reduced to about 8 mm. Shortness of breath at rest often develops when the airway diameter is about 5 mm, and stridor is now often present. Stridor is a loud, musical sound of constant pitch that usually indicates the presence extrathoracic airway obstruction most often affecting the larynx or upper trachea.
Unlike wheezing, which is a feature of diffuse peripheral airway obstruction and occurs primarily during expiration, stridor usually occurs during inspiration and is loudest in the neck. The sound can usually be appreciated without using a stethoscope. Although one would expect to be able to distinguish stridor from wheezing coming from lower airways, sound recordings from the neck and chest have shown that the sounds from the asthmatic wheeze and stridor have similar frequencies. Hence there may be errors in diagnosis and a UAO caused by a tumor or foreign body may be mistakenly treated as asthma.
Breathing maneuvers that increase inspiratory airflow, such as forced inspiration or voluntary hyperventilation, accentuate the intensity of stridor. Neck flexion may change the loudness of stridor. When the obstructing lesion is fixed, both inspiratory and expiratory stridorous sounds may be heard. At times, the character of a patient’s voice may be a clue to the presence of UAO. Hoarseness may be a sign of a laryngeal abnormality. Muffling of the voice may be present when vocal cords are paralyzed.
LABORATORY ASSESSMENT
Just as UAO must be quite advanced before development of symptoms, physiologic abnormalities do not become apparent on lung function testing until severe obstruction occurs. Studies of subjects breathing through tubes of varying diameters suggest that UAO must narrow the airway lumen to less than 8 mm in diameter to produce abnormalities on a flow–volume loop. This corresponds to an obstruction of more than 80% of the normal median diameter of the tracheal lumen. The forced expiratory volume in 1 second (FEV1) remains above 90% of control until a 6-mm orifice is created. Therefore, spirometry, which is often the first screening test for pulmonary symptoms, may not be an effective way to detect upper airway abnormalities. The peak expiratory flow rate (PEFR) and maximal voluntary ventilation (MVV) are more sensitive than the FEV1 in detecting UAO. Of note, a noncritical airway may become so with a lesser degree of obstruction in the context of airway secretions, edema, or bleeding.10
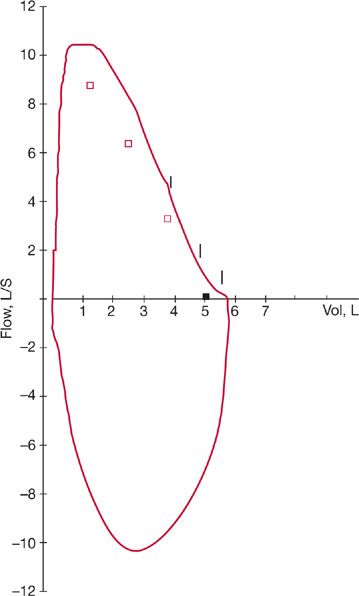
The flow–volume loop, which is a recording of maximal inspiratory and expiratory flows at various lung volumes, is an important tool for the diagnosis of UAO. The configuration of the normal flow–volume loop is shown in Figure 49-2. During a forced expiratory maneuver from total lung capacity (TLC), the maximal flow achieved during the first 25% of the forced vital capacity is dependent on effort, that is, an increase in driving pressure (effort) may result in increased flow. During the remaining 75% of the forced vital capacity maneuver, flow is determined by the mechanical properties of the lungs and is not effort dependent. During this portion of forced exhalation, a linear deceleration of flow is caused by dynamic compression of the intrathoracic airways (Fig. 49-3A). An increase in effort and therefore pleural pressure causes further compression of the intrathoracic airways and a further limitation of airflow. At higher lung volumes, flow may be limited by a UAO. At low lung volumes, flow may not be affected by a UAO, since measurement of flow in this effort-independent portion of the curve represents the function of the peripheral airways. Since the FEV1 reflects a large portion of flow at these lower lung volumes, it is not a sensitive test for UAO. Because the PEFR reflects flow at higher lung volumes, it may be abnormal when the FEV1 is not.
Figure 49-2 Normal flow–volume loop following maximal expiratory (above) and inspiratory (below) effort. Small vertical lines denote seconds.
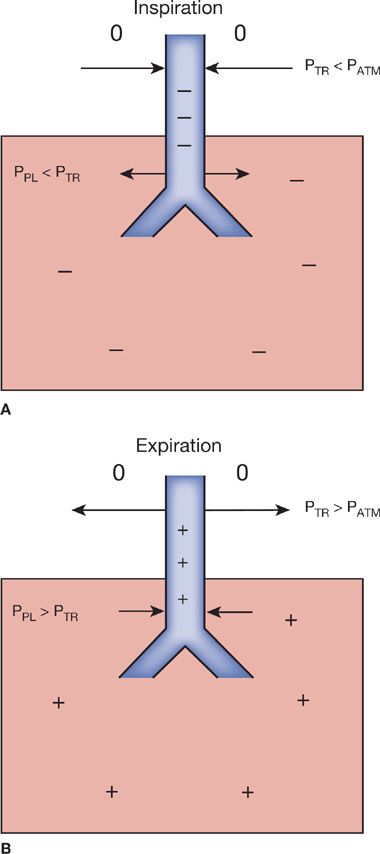
Figure 49-3 Forces acting on intra- and extrathoracic airway walls during inspiration and expiration. 0, atmospheric pressure; +, positive pressure; –, negative pressure. A. During inspiration, extrathoracic tracheal pressure (PTR) falls below atmospheric pressure (PATM), favoring narrowing of the lumen (arrows). Intrapleural pressure (PPL) becomes negative, favoring airway enlargement (arrows). B. During expiration, the extrathoracic tracheal pressure (PTR) becomes positive and, therefore, greater than PATM, favoring enlargement of the lumen (arrows). Intrapleural pressure (PPL) is positive, causing dynamic compression of the intrathoracic trachea (arrows).
In generating the flow–volume loop, forced inspiratory flow is limited by effort during the entire inspiratory maneuver. Flow increases from residual volume to near the midportion of the curve, where it becomes maximal at the peak inspiratory flow rate. Flow then declines until TLC is reached. The pressure surrounding the extrathoracic portion of the upper airway is atmospheric. The turbulent nonlaminar airflow, which occurs during forced inspiration and causes airway pressure to fall in this portion of the airway, favors slight narrowing of the extrathoracic airway (Fig. 49-3B). Peak inspiratory flow, therefore, is less than peak expiratory flow in normal subjects. Because of the dynamic compression of the intrathoracic airways that occurs during exhalation, flow during the middle of inspiration, that is, the forced inspiratory flow at 50% of the forced vital capacity (FIF50%), is usually greater than flow during the middle of forced expiration, that is, the forced expiratory flow at 50% of the forced vital capacity (FEF50%). Typical patterns of the flow–volume loop may be seen, depending on whether the obstruction to flow is “fixed” or “variable,” and whether the site of the obstruction is above or below the thoracic outlet or suprasternal notch.
Fixed obstructions of the upper airway are those in which the cross-sectional area does not change in response to transmural pressure differences during inspiration or expiration. A fixed obstruction may occur in either the intrathoracic or extrathoracic airways. Irrespective of the site of the obstruction, a fixed lesion results in the flattening of the inspiratory and expiratory phases of the flow–volume loop. A variable obstruction is one that responds to transmural pressure changes, eliciting varying degrees of obstruction during the respiratory cycle. Since the stresses on the intrathoracic and extrathoracic airways are different, changes seen in the flow–volume loop vary according to the site of the obstruction.
A number of conditions have been associated with nondistensible narrowing of the upper airway and fixed airway obstruction. Benign strictures and malignancy are common examples. Maximal inspiratory and expiratory flow–volume loops with fixed obstruction show constant flow, represented by a plateau during both inspiration and expiration (Fig. 49-4A,B). On the expiratory curve, the plateau effect is seen in the effort-dependent portion of the curve near TLC; very little change is noted in the effort-dependent portion near residual volume. Since the inspiratory curve is similar in appearance, the ratio of FEF50% to FIF50% is normal (close to 1). The forced inspiratory volume in 1 second (FIV1) and FEV1 are nearly the same in fixed UAO.
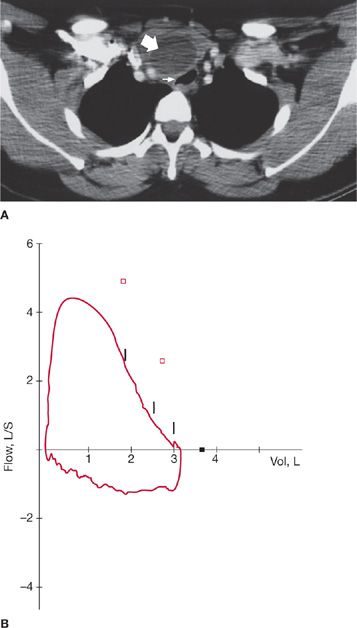
Figure 49-4 A, B. Flow–volume loop in fixed upper airway obstruction due to laryngeal abscess in a 56-year-old man who developed persistent wheezing, hoarseness of voice, and intermittent stridor for 3 months after a brief intubation for asthma exacerbation. Computed tomography scan of the neck (A) shows a laryngeal abscess with significant impingement on the laryngeal inlet. The flow–volume loop (B) demonstrates a plateau of flow during inspiration and expiration; the FEF50%/FIF50% ratio is near 1.
Unilateral vocal cord paralysis is a common cause of variable extrathoracic obstruction. A variable extrathoracic airway obstruction increases the turbulence of inspiratory flow, and intraluminal pressure falls markedly below atmospheric pressure. This leads to partial collapse of an already narrowed airway and a plateau in the inspiratory flow loop (Fig. 49-5A,B). Expiratory flow is not significantly affected, since the markedly positive pressure in the airway tends to decrease the obstruction. The ratio of FEF50% to FIF50% is high (usually greater than 2). Similarly, the FEV1 is greater than the FIV1.
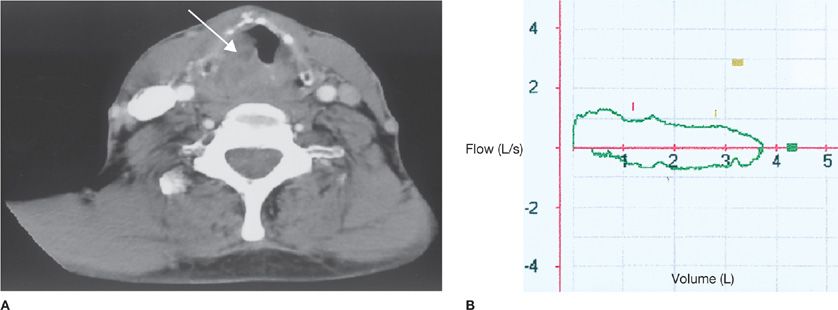
Figure 49-5 Variable extrathoracic obstruction due to thyroid cyst in a 32-year-old woman with dyspnea on exertion. A. Computed tomography of the neck shows a 10- × 4-cm cystic mass (large arrow) in the thyroid gland compressing the trachea (small arrow). B. Flow–volume loop shows inspiratory obstruction. FEF50%/FIF50% is very high, and the inspiratory curve is flattened.
A variable obstruction in the intrathoracic airways reverses the situation. A predominant reduction in maximal expiratory flow is associated with a relative preservation of maximal inspiratory flow. This association occurs because intrapleural pressure becomes decidedly positive during forced expiration and causes dynamic compression of the intrathoracic airways. The obstruction caused by an intrathoracic lesion is accentuated and a plateau in expiratory flow occurs on the flow–volume loop (Fig. 49-6A,B). A plateau of flow suggests that the lesion has caused the airway lumen to reach its minimal size. A flow peak may precede the plateau, suggesting that the obstruction may not affect flow until a certain lung volume is reached. During inspiration, intrapleural pressure is markedly negative; therefore, the obstruction is decreased. The ratio of FEF50% to FIF50% is very low and may approach 0.3. Similarly, the FEV1 is considerably lower than the FIV1. Although the flow ratios are similar to those seen in patients with COPD and chronic asthma, these disorders are distinguished from UAO by the appearance of the flow–volume loop. Thus, the expiratory curve in patients with COPD and asthma is primarily altered in the effort-independent portion of the curve, leading to a characteristic shape unlike the plateau configuration of a UAO (Fig. 49-7).
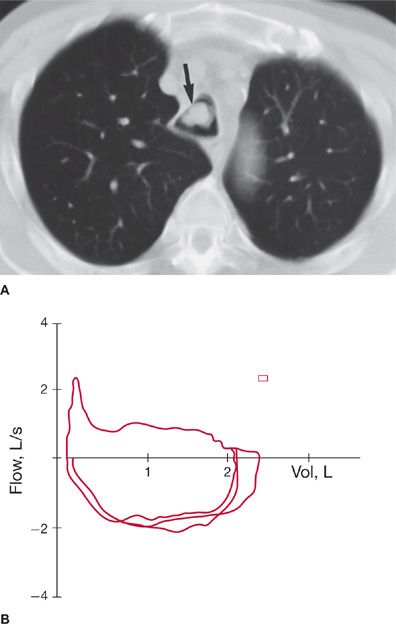
Figure 49-6 Variable intrathoracic obstruction due to squamous cell carcinoma of the trachea. A. Computed tomography of the chest shows a tracheal lesion (arrow), which was not readily apparent on plain chest radiograph. B. Superimposed flow–volume loops show a plateau of expiratory flow preceded by a peak of flow at higher lung volumes. The forced inspiratory flow is preserved in comparison to expiratory flow, but it is also reduced. FEF50%/FIF50% is 0.4.
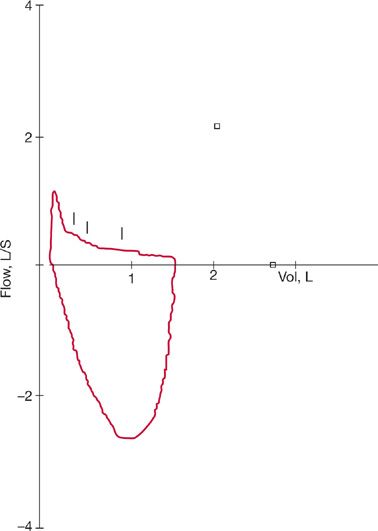
Figure 49-7 Flow–volume loop typical of chronic obstructive lung disease. Very low FEF50%/FIF50% and typical curvilinear shape are noted.
When a hospital laboratory or physician’s office is not equipped to perform flow–volume loops, results of other tests, such as routine spirometry, may be helpful. If the forced spirogram shows that the PEFR is reduced disproportionately to the reduction in FEV1, a UAO should be suspected. Other findings that suggest the diagnosis include a ratio of less than 1.0 for the inspiratory flow between 25% and 75% of the inspired vital capacity (FIF25–75%) and a value of less than 1.0 for the expiratory flow between 25% and 75% of the expired vital capacity (FEF25–75%). Another indication is an FEV1 that is decreased to the same degree as the FEF25–75%. The MVV may also be a useful test, since it measures both inspiratory and expiratory flows. A ratio of MVV to FEV1 of less than 25% is often found with UAO. Whenever the MVV is reduced in association with a normal FEV1, a diagnosis of UAO should be considered.
In contrast to the situation in patients with diffuse obstructive disease of the lower airways (e.g., COPD, asthma), the distribution of ventilation in the lungs is normal, and ventilation–perfusion mismatch does not occur. Hypercarbia is not seen unless the degree of obstruction is very severe, although nocturnal hypercarbia may occur while daytime levels of PCO2 are normal. Hypoxemia is also not present except during exercise and with severe airflow limitation, when it may accompany increases in the level of PCO2. In contrast to asthma and many instances of COPD, the airflow obstruction caused by an upper airway lesion does not resolve following the inhalation of a bronchodilator unless there is also a component of small airway disease that is often the case.
IMAGING
When acute airflow obstruction occurs as a result of an abnormality of the extrathoracic airway, roentgenographic studies of the soft tissues of the upper chest and neck in the emergency setting may be helpful (Fig. 49-8). However, computed tomography (CT) has afforded the most important approach to imaging of the extrathoracic airways (Fig. 49-9). The standard chest roentgenogram is often not helpful in detecting the presence, or the cause, of UAO. Occasionally, in patients with chronic airway obstruction, generalized hyperinflation of the lungs may occur; in the absence of asthma or COPD this finding may raise suspicion of occult disease in the central airways. The trachea is usually well visualized on the posteroanterior (PA) and lateral views in chest roentgenograms of good quality. It is located in the midline and is moderately deviated at the level of the aortic arch. However, many standard roentgenograms are underpenetrated so that the trachea may become a “blind spot.” In one study, only 13 of 53 tracheal tumors were evident to the radiologist on the standard PA roentgenogram. The use of digital imaging techniques may avoid such pitfalls. However, thoracic CT studies have become the procedure of choice for imaging the upper airway.
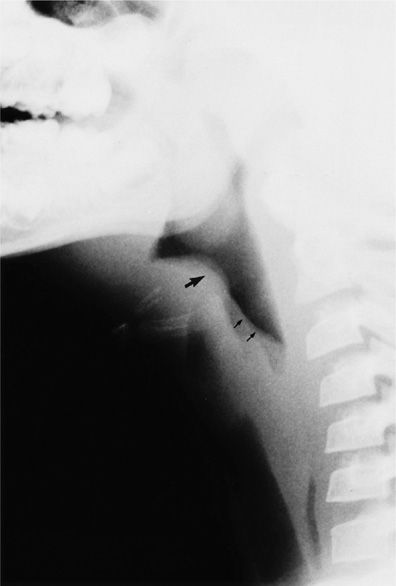
Figure 49-8 Acute epiglottitis. Lateral soft tissue radiograph of the neck of a patient with stridor shows swelling of the epiglottis (large arrow) and loss of normal convexity of the edematous aryepiglottic folds (small arrows).
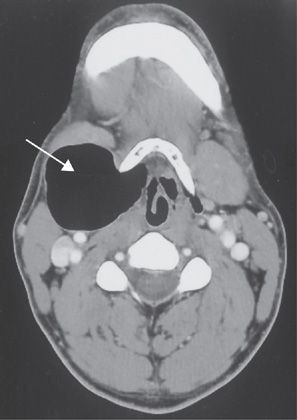
Figure 49-9 Computed tomography scan of the neck demonstrating a large laryngocele compressing the lateral wall of the larynx (arrow) causing positional air flow obstruction.
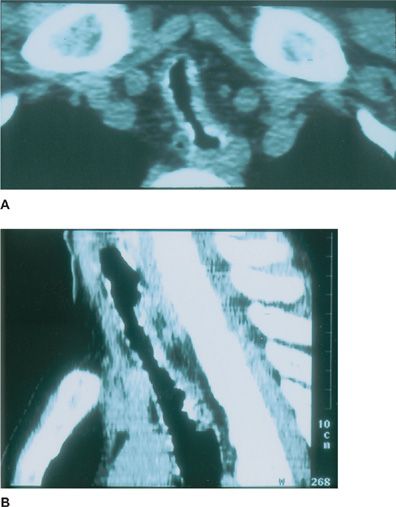
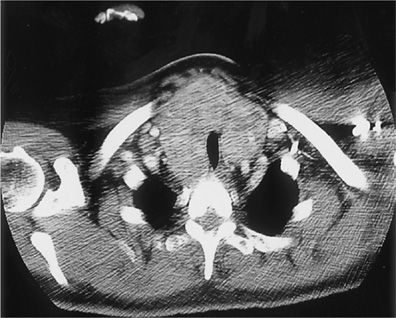
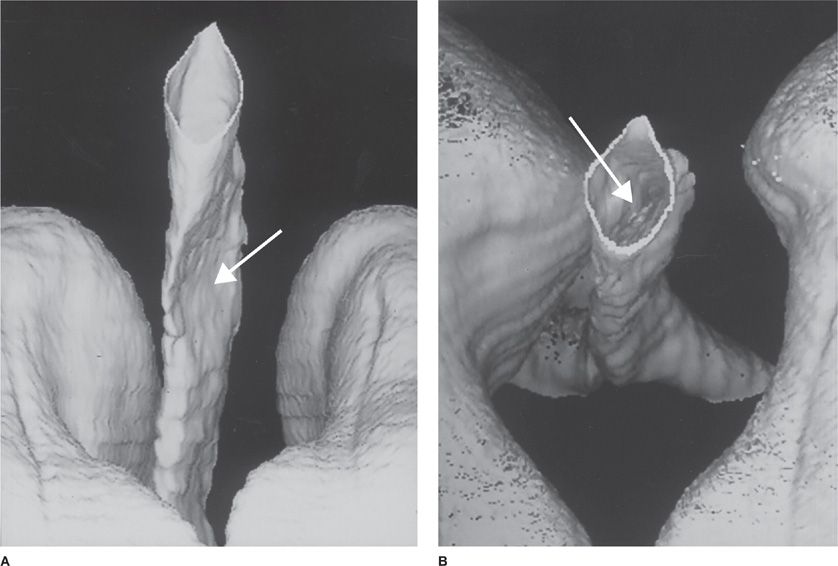
The sensitivity of CT scanning for detecting upper airway disease surpasses that of the routine chest roentgenogram (97% vs. 66%, respectively). Helical CT (HCT) scanning minimizes artifacts due to respiratory motion and provides imaging of the whole thoracic volume during a single breathhold.11 The technique represents an improvement over conventional CT scanning in that it allows detection of intraluminal, submucosal, and extraluminal lesions (Figs. 49-10A,B and 49-11). Since the early 1990s, HCT has become the preferred noninvasive modality for evaluation of the central airways. The use of HCT using multidetector technology and thin collimation provides high-resolution images of the entire thorax, improved special resolution, greater speed of image acquisition, and excellent contrast enhancement. HCT techniques using multiplanar and three-dimensional reconstruction can provide virtual images of the thorax that enhance the perception of local and diffuse anatomic lesions of the upper airways (Fig. 49-12). The images may demonstrate the degree of tracheal widening or narrowing, show the location and longitudinal extent of abnormalities, assess tracheal wall thickness, and demonstrate associated extratracheal diseases.
Figure 49-10 A. Computed tomography scan of the chest demonstrating marked narrowing of the trachea with intraluminal calcified nodular projections in a patient with tracheopathia osteoplastica. B. Computed tomography scan of the chest demonstrating multiplanar reformation of the trachea in the sagittal plane of the same patient.
Figure 49-11 Computed tomography scan of the chest demonstrating marked extraluminal compression of the trachea caused by intrathoracic goiter.
Figure 49-12 Helical computed tomography scan of the chest with three-dimensional reconstruction of the upper airway showing focal tracheal compression (arrows) (A, B.)
The use of paired inspiratory dynamic and expiratory multislice HCT has proved helpful for the diagnosis of tracheomalacia. Because the maximal degree of collapse in tracheomalacia usually occurs during exhalation rather than at end expiration, dynamic expiratory imaging is preferable to end-expiratory imaging. If complete collapse is not demonstrated during expiration, then one should confirm the diagnosis by quantitatively measuring the degree of airway luminal narrowing during expiration. Tracheomalacia is generally defined as a reduction in cross-sectional area of greater than 50% on expiratory images; however, this cut point may not be discriminatory enough to detect clinically significant tracheomalacia.12
Another novel CT-based imaging technique is virtual bronchoscopy. The use of volumetric imaging allows for an intraluminal three-dimensional reconstruction of the airways and surrounding tissues. The technique has been used with a high degree of accuracy in assessing the width, length, and contour of fixed airway lesions, but it has not been effective in defining dynamic airway lesions, such as excessive dynamic airway collapse.
Magnetic resonance imaging (MRI) is another modality that may be used to assess the central airways and surrounding mediastinal structures. MRI provides a multiplane image of the chest without the need for contrast material. However, the technique is best used to investigate vascular structures surrounding central airways, such as vascular rings or aneurysms that may compress the trachea, rather than the airways themselves, which are better visualized using CT scanning.
ENDOSCOPY
Bronchoscopy and direct laryngoscopy provide a real-time morphologic and functional evaluation of the upper airway. Imaging techniques are often limited by their inability to document the dynamic nature of many airway lesions and may underestimate the extent of an obstruction.
With respect to laryngeal disease, endoscopy allows the operator to determine whether the vocal cords are mobile or fixed in abduction or adduction or alternatively moving paradoxically. This valuable information can help guide management. In cases of traumatic or thermal injury to the airways, edema, laryngeal fracture, or carbonaceous materials can be readily identified.
CAUSES OF UPPER AIRWAY OBSTRUCTION
UAO may be observed in a wide variety of settings, including infections, trauma (including iatrogenic), vascular disorders, and a wide variety of benign and malignant tumors. Each is discussed below.
 INFECTION
INFECTION
A broad variety of infections may eventuate in UAO.
Deep Cervical Space Infections
Deep cervical space infections occur in potential spaces bounded by the deep cervical fascia. The cervical fascia is divided into a superficial and, a more complex, deep layer. This configuration and complexity divides the neck into functional units. Infection can spread along the planes formed by the cervical fascia. Infections affecting the deep neck tissues may result in life-threatening UAO.
Patients with deep cervical space infections may present with sore throat, odynophagia, neck swelling, pain, fever, and dyspnea. Stridor and profound respiratory difficulty are signs of significant UAO. Parapharyngeal, peritonsillar, submandibular, and retropharyngeal abscesses appear to be common locations in adults. The bacteriology and initiating event of deep cervical infections appear to have changed over time.
Mixed infections caused by aerobic and anaerobic infections are common and have been reported in up to two-thirds of cases.13 Streptococcus viridans and Klebsiella pneumonia are common pathogens. Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pneumoniae, Escherichia coli, and Haemophilus influenzae are other agents that are commonly reported. Alpha and beta hemolytic streptococci appear to have significantly declined in frequency. Overall, an odontogenic origin is probably most common, with upper respiratory tract infections as an important etiology in children. Intravenous drug abuse, mandibular fractures, iatrogenic and noniatrogenic traumatic injury to the upper airway, underlying malignancy, and poor underlying immune status are associated conditions. Ludwig’s angina – an infection of the submandibular space and the floor of the mouth – is potentially lethal and is commonly associated with significant UAO. This entity is usually a cellulitic process and can affect the submandibular spaces bilaterally. Many cases with true Ludwig’s angina require tracheostomy.
Treatment of deep cervical infections involves maintenance of oxygenation and ventilation by securing an adequate airway, administration of appropriate antibiotics, and when indicated, use of surgical drainage. Complications of deep cervical infections include UAO, Lemierre’s syndrome (see next paragraph), distant infection, septic embolization, carotid artery rupture, pulmonary embolism, direct extension of infection resulting in mediastinitis and empyema, and rupture of the abscess during intubation or other interventions.14
One particularly virulent cervical infection, known as Lemierre’s syndrome, arises from a nasopharyngitis or peritonsillar abscess. This lateral pharyngeal space infection results in suppurative thrombophlebitis of the internal jugular vein, septicemia, and metastatic abscess formation, particularly in the lungs and joints. Fusobacterium necrophorum is usually the causative agent and has been cultured from blood in over 80% of cases. Symptoms begin with a sore throat, fever, and painful swelling in the neck, followed by tender lymphadenopathy and tenderness along the sternocleidomastoid muscle (representing thrombophlebitis of the internal jugular vein). Dysphagia, trismus, and UAO may occur as a result of swelling of the lateral pharyngeal space. Contrast-enhanced CT scan of the neck is most useful in establishing the diagnosis of thrombosis of the internal jugular vein and may demonstrate soft tissue abscesses, fasciitis, and myositis, which may require extensive surgical debridement. Without the use of early and appropriate antibiotics, such as high-dose penicillin with metronidazole, or monotherapy with clindamycin, the mortality rate approaches 100%.
Epiglottitis
Epiglottitis is an infectious process that causes variable degrees of inflammation and edema of the epiglottis and supraglottic structures. Supraglottitis may be more appropriate term in adults, since the supraglottic structures usually are involved with variable involvement of the epiglottis. This condition can be life-threatening. Its prevalence is 0.18 to 9.7 cases per million adults; the mortality rate may be as high as 7.1%. Clinical presentation includes odynophagia, with inability to swallow secretions, sore throat, dyspnea, hoarseness, fever, tachycardia, and stridor. In one review, 44% of the patients had a normal routine oropharyngeal examination.15
Fiberoptic laryngoscopy is necessary to make the diagnosis. The procedure is safe in adults with suspected epiglottitis and should be done without delay. Radiographic studies can be helpful in ruling out other etiologies with similar presentations and in evaluating potential complications. However, the airway must be secured, and radiographic studies should not delay diagnosis or management.
Supraglottitis may involve the base of the tongue, uvula, pharynx, and false vocal cords. The disease may be increasing in prevalence among adults and declining in children, perhaps, reflecting introduction of Haemophilus b conjugate vaccines. Young adult males are commonly affected. The disorder appears to be more prevalent in colder, winter months and in smokers. Blood cultures are positive in less than one-third of cases. Although Haemophilus influenzae is the most common organism isolated in children, adult supraglottitis may be caused by a variety of organisms, including Haemophilus influenzae, pneumococci, group A streptococci, S. aureus, Streptococcus viridans, a variety of anaerobic organisms, mycobacteria, fungi, and viruses. Throat cultures can be helpful in diagnosis and management; however, treatment should not be delayed while awaiting culture results.
Illicit drug use may be associated with epiglottitis, with inhalation of heated objects (e.g., metal pieces from a crack cocaine pipe or the tip of a marijuana cigarette) causing thermal injury to supraglottic structures. Signs, symptoms, and roentgenographic and laryngoscopic findings are similar to infectious epiglottitis.
Initial antibiotic therapy using a third-generation cephalosporin or extended-spectrum penicillin is reasonable. The prevalence of resistant organisms should be taken into account when choosing empiric antibiotic coverage. Corticosteroids often are used in management of acute epiglottitis despite lack of evidence to support their use. Based on anecdotal case reports, epinephrine is also used.
Patients should be observed closely and experienced staff should be available immediately to secure the airway by intubation or surgical approach, if needed. Securing the airway is extremely important in patients who develop stridor and other signs of significant airway obstruction. Mortality in this group has been reported to be as high as 17.6%.16
Laryngotracheobronchitis and Bacterial Tracheitis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree