they may be related to increased concentrations of activated macrophages and T lymphocytes at the plaque shoulder.
TABLE 10.1. Causes of unstable angina and non-Q-wave MI | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||
TABLE 10.2. Three principal presentations of unstable angina | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
and vascular emergencies). Serial examinations may uncover signs such as hypotension, brady/tachycardia, pulmonary rales, a third heart sound, or a new or worsening murmur that predict higher risk and demand a more aggressive management (diagnostic and treatment) pathway (12).
TABLE 10.3. Likelihood that signs and symptoms represent an ACS secondary to CAD | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||
depression (15). All these ECG findings may be transient phenomena, and this illustrates the importance of serial tracings. Continous ST-segment monitoring may reveal episodes of otherwise undiagnosed ischemic episodes.
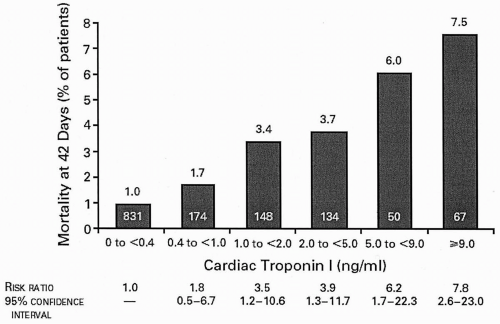
acute coronary syndromes (ACSs) continues to elicit vigorous research interest. C-reactive protein (CRP) levels in ACS appear to be related to long-term mortality in an independent and additive fashion to troponin levels (19). The role of other inflammatory markers, such as amyloid A and interleukin-6, in the day-to-day management of patients with ACS, is as yet undefined.
TABLE 10.4. Noninvasive risk stratification | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||
intraventricular conduction delay, paced rhythm, preexcitation, and digoxin effect. Echocardiographic or nuclear stress imaging should be added in patients with ECG abnormalities that prevent accurate interpretation. Pharmacologic stress testing can be performed in patients who cannot achieve an adequate exercise stress on the treadmill.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree