Univentricular Atrioventricular Connection
Michael G. Earing
Donald J. Hagler
William D. Edwards
Nomenclature
Considerable controversy exists regarding the definition, classification, and nomenclature for various forms of complex congenital heart disease. As a result, the potential exists for confusion and misunderstanding both between and within institutions. This is especially true for the nomenclature of hearts with a large dominant ventricle and a small rudimentary ventricle. Terms that have been used to describe this group of hearts include single ventricle, univentricular heart, common ventricle and, more recently, single functional ventricle.
Van Praagh et al. (1) have emphasized that it is inaccurate to label hearts in this category as “single ventricle,” given that there are always two-ventricular chambers present in mammalian hearts. In the late 1970s and early 1980s, the early work of Anderson et al. (2,3) attempted to clarify the classification of these hearts by introducing a new definition for what constitutes a ventricle. This definition was based on several important observations. First, normal ventricles possess an inlet, trabecular, and outlet portion. The inlet portion extends from the atrioventricular (AV) annulus to the insertions of the papillary muscles and does not require a perforate AV valvar annulus. The trabecular portion exists between the inlet and outlet portions and includes the ventricular apex. Finally, the outlet portion of the ventricle includes the nontrabeculated region beneath a semilunar valve. The second observation upon which Anderson et al. based their definition was that in the normal heart the inlet and outlet portions of the morphologic left ventricle (LV) are in fibrous continuity. But, in a morphologic right ventricle, the inlet and outlet portions are separated from one another by the crista supraventricularis. Their final observation for their definition of a ventricle was that in the normal heart, each trabecular zone receives its own inlet. Based on these observations, Anderson et al. initially proposed that a chamber must have 50% or more of an inlet portion to be classified as a ventricle and need not have an outlet portion. Chambers receiving <50% of an inlet were defined as rudimentary chambers. Rudimentary chambers consisting of an outlet portion were classified further as an “outlet chamber.” Those possessing only a trabecular zone were called “trabecular pouches.” Based on these rules, Anderson et al. used the term univentricular heart of left ventricular type to describe hearts in which the dominant chamber was morphologically an LV and the rudimentary chamber had characteristics of the trabecular portion of a right ventricle. They used the term univentricular heart of the right ventricular type to describe hearts in which the dominant chamber was morphologically a right ventricle and the rudimentary chamber had the characteristics of the trabecular portion of an LV.
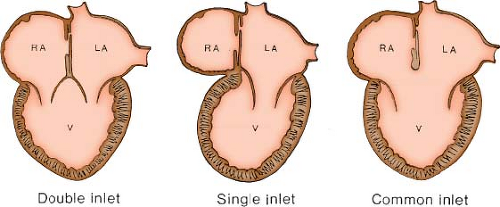
While their definitions have been accepted by many, other investigators believed these definitions were confusing, arguing that using the term “univentricular heart” is misleading because all hearts have two ventricular chambers. As a result, in the late 1970s and early 1980s, many other authors continued to describe these hearts based upon their abnormal AV connection, and preferred terms such as double-inlet LV and tricuspid atresia. In 1984, in response to arguments against their definition of a ventricle, Anderson et al. (2) introduced the term “univentricular AV connection” to describe hearts in which the atria are joined to only one ventricle, either because one of the connections between the atria and the ventricular mass is absent (absent right or left AV connection) or because both atria are committed to the same ventricle (double-inlet ventricle). In this system, the emphasis is on the use of the sequential segmental approach to define the dominant ventricular morphology present, the position and morphology of the rudimentary ventricle, the type of AV connection, and, finally, the position of the semilunar valves.
There are six possible AV connections. Two are biventricular (concordant and discordant), one is ambiguous (in cases of atrial isomerism), and two are univentricular (double-inlet right or LV and absent right or left AV connection). We prefer the definition of a ventricle as an endocardial-lined chamber within the ventricular mass, regardless of the ventricular components that are present or absent. The term “single functional ventricle” encompasses hearts with a small nonfunctional ventricle and includes hearts with univentricular AV connections, as well as those with an atretic semilunar valve and an intact ventricular septum (the hypoplastic left and right heart syndromes). Patients with any type of single functional ventricle are amenable to a modified Fontan type of surgical repair.
Throughout the remainder of this chapter we will use the sequential segmental approach to discuss conditions resulting in a univentricular AV connection. For each condition, we will describe the dominant ventricular morphology as left or right, the type of AV connection, the position and morphology of the hypoplastic ventricle, and the spatial relationship of the great arteries. We will use the term “single functional ventricle” to help avoid any conceptual confusion that terms like “univentricular heart” and “single ventricle” might cause.
Pathologic Anatomy
Ventricular Morphology
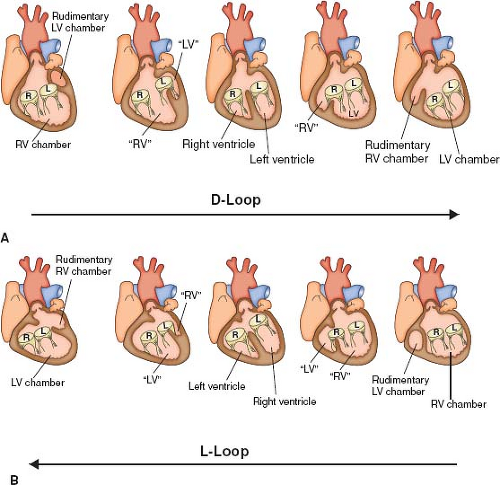
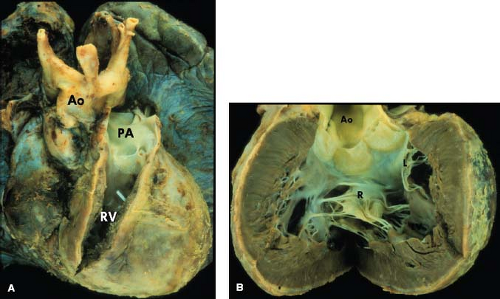
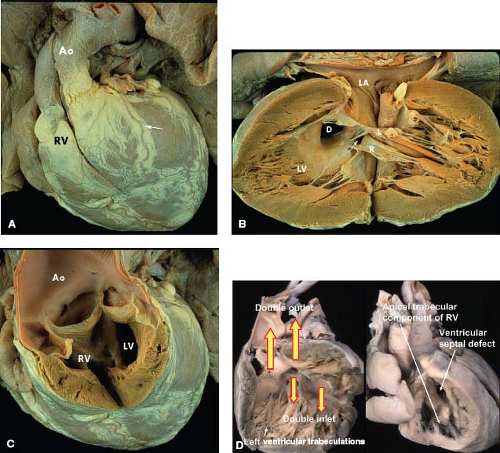
The most common form of univentricular AV connection is a double-inlet LV with a hypoplastic right ventricle (1). In this anomaly, the rudimentary right ventricle is located anterior and superior to the dominant left ventricular chamber. Most commonly the dominant LV is connected to the pulmonary trunk and the rudimentary right ventricle, to the aorta (discordant ventriculoarterial [VA] connections). If the hypoplastic right ventricle lies along the right shoulder of the heart, the sidedness of the dominant LV will be normal, and if it is found on the left shoulder of the heart, then the left ventricular sidedness will be the mirror image (l-loop ventricles). In the dominant chamber, direct continuity between one or both AV valves and the semilunar valve establishes its morphology as left ventricular. In short axis, the plane of the ventricular septum will be angled rather than perpendicular to the diaphragmatic ventricular wall and it will be displaced superiorly.
Double-inlet right ventricle is an exceedingly rare anomaly. In this condition, the hypoplastic left ventricular chamber will be located along the inferior (diaphragmatic) aspect of the heart, usually to the right or left of midline. Only rarely will the hypoplastic chamber give rise to a great artery. Consequently, the VA connections are almost always right ventricular and may be double outlet, common outlet (truncus arteriosus), or single outlet (with pulmonary or aortic atresia). Demonstration of separation of the AV valves from the semilunar valves by a collar of myocardium (the conus, infundibulum, or outflow tract) will establish the morphology of the dominant chamber as right ventricular.
Rarely, the morphology of neither the dominant chamber nor the hypoplastic chamber can be determined with certainty. Such cases are typically categorized as single functional ventricle of undifferentiated or indeterminate type.
Atrioventricular Connections
A double-inlet AV connection is usually associated with a dominant morphologic LV. In this situation, both atria connect to the dominant ventricle usually by two distinct AV valves, which are usually mirror-image morphologic mitral valves, thus fulfilling the embryologic concept that AV valve morphology always corresponds to the morphology of the ventricle to which they connect (Fig. 50.1). When one of the valves straddles into the hypoplastic right ventricle, it will have a hybrid mitral–tricuspid morphology. In such hearts, the AV valves should be labeled as “right” and “left” rather than ambiguously as “tricuspid” and “mitral.”
In some hearts, usually in which the dominant ventricle is of right ventricular morphology, there may be double-inlet ventricle via a common rather than via two separate AV valves. These hearts are classified as “double inlet” in that more than 50% of the connection between each atrium and the ventricular mass is to the same ventricle, thus emphasizing that the term “double inlet” refers to the nature of sequential connection, rather than the nature of the valves guarding the AV junction. It should be noted that double-inlet (usually right) ventricle via a common AV valve occurs much less frequently than double-inlet LV (12% vs. 88%) (4).
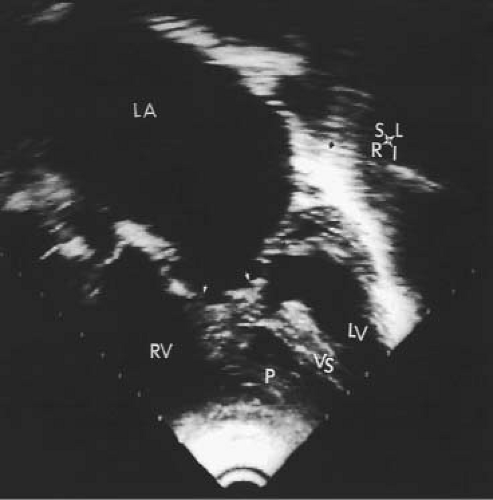
The third form of univentricular AV connection is one in which there is absence of one AV connection, which includes tricuspid atresia and mitral atresia (discussed in detail in Chapters 38 and 46). These hearts must be distinguished from those in which there is an imperforate valve orifice (Figs. 50.2 and 50.3).
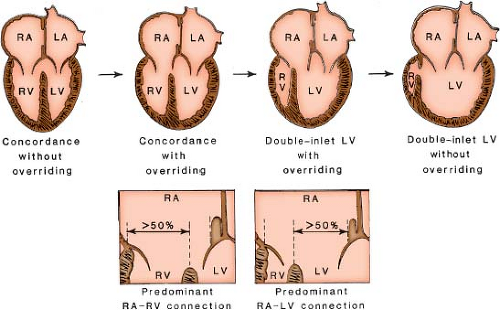
Annular, rather than cordal, features are used to determine AV connections. Overriding refers to partial commitment of an AV valve annulus to the contralateral ventricular chamber and results from malalignment of the atrial and ventricular septa. It can influence the determination of an AV connection. The percentage of annular commitment to a ventricular chamber relative to the position of the ventricular septum determines the AV connection to a ventricular chamber. In the setting of an overriding AV valve, an atrium is considered to connect to the ventricle into which 50% of its valve empties. Thus, an annular commitment of 50% or more
to a ventricular chamber establishes the valve connection to that ventricular chamber (Fig. 50.4).
to a ventricular chamber establishes the valve connection to that ventricular chamber (Fig. 50.4).
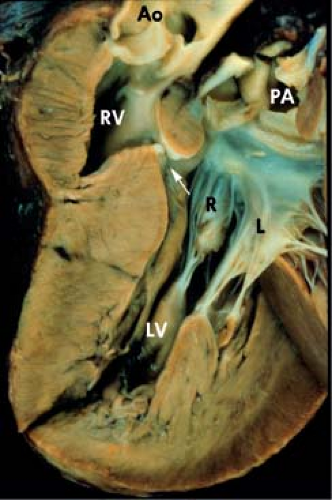
In contrast to annular overriding, straddling is a feature of the tendinous cords and papillary muscles and results from their insertions into the contralateral ventricle through a ventricular septal defect (VSD). In double-inlet LV, an AV valve may straddle into the hypoplastic right ventricular chamber. The valve that straddles is almost always on the same side as the hypoplastic outlet chamber. Thus, with a right-sided hypoplastic right ventricle, it is the right AV valve that may straddle. Figure 50.5 schematically depicts the progressive changes in AV connections that occur, linking double-inlet connection to overriding connections to normal concordant connection. This may occur with either d-loop or l-loop ventricles. Straddling
AV valves are often large and redundant and can develop appreciable regurgitation.
AV valves are often large and redundant and can develop appreciable regurgitation.
Ventriculoarterial Connections
In univentricular heart, any form of VA connection can occur, including concordant connections (i.e., aorta arising from morphologic LV and pulmonary trunk from RV), discordant connections (i.e., aorta arising off rudimentary outlet chamber with right ventricular morphology), double outlet from the dominant ventricle or from the hypoplastic rudimentary ventricle, and single outlet from the dominant ventricle. Similar to the normal two-ventricle heart, the VA relationship is defined by the ventricle from which most (>50%) of an overriding semilunar valve originates.
Although many types of VA connections can occur in the setting of the univentricular heart, certain combinations are more common than others. In the setting of univentricular heart of left ventricular morphology, the majority of patients will have discordant VA connections, with the aorta arising from the rudimentary right ventricle, and the pulmonary artery arising from the dominant LV. In the series by Shiraishi and Silverman (4), 86% of patients with univentricular LVs with a double-inlet AV connection had transposition of the great arteries (4). Similar to the series published by Van Praagh et al. (1), the majority of patients in their series who had transposition of the great arteries also had l-looped ventricles (occurring in 63% of the patients). In this form, the aorta arises from the rudimentary outlet chamber of right ventricle morphology that is anterior and leftward of the AV valves. The pulmonary artery arises from the morphologic LV. For the rest of the patients in their series, 23% were found to have transposition of the great arteries of the d-loop variety (i.e., aorta arising from outlet chamber of right ventricle morphology that sits anterior and rightward) and 14% of the patients had normally related great vessels (Holmes heart). Other common combinations described in the literature include the pattern seen with univentricular heart of right ventricular morphology. In this situation, the VA connection usually is double outlet from the dominant right ventricle or is a single outlet with pulmonary atresia. In the univentricular heart of indeterminate morphology, as there is truly a single ventricle there can only be a double outlet or a single outlet.
Pulmonary outflow tract obstruction frequently occurs in hearts with a univentricular AV connection. This can occur with either concordant or discordant ventriculoarterial connections. When subpulmonary obstruction occurs, it typically is present within the ventricle of left ventricular morphology and is due to posterior deviation of the infundibular septum. Other causes of pulmonary outflow tract obstruction include anomalous attachments of the right AV valve or herniation of valvular tissue into the pulmonary outflow tract. In rare cases, there can be severe stenosis at the level of the pulmonary valve due to malformed leaflets or annulus hypoplasia.
Interventricular Communications
The connection between the dominant and rudimentary ventricles has been referred to by several terms in the literature, including VSD, the bulboventricular foramen, and the outlet foramen. In double-inlet LV, the defect will be in the muscular portion of the trabecular septum in most cases, separated from the semilunar valves and completely surrounded by muscle. However, when there is hypoplasia of the infundibulum, the defect may extend into the outlet septum to sit immediately underneath the semilunar valve (subaortic VSD). The communication often can be restrictive, occurring in 47% of cases in the series published by Bevilacqua et al. (5). Even when it is unrestrictive at birth, the defect will often become restrictive over time. In the series by Bevilacqua et al. (5), patients with double-inlet left ventricle (DILV) and transposed great arteries and pulmonary stenosis, the communication was rarely restrictive. However, when transposition of the great arteries was present without pulmonary stenosis, a restrictive outlet foramen was significantly more common.
Common Forms of Univentricular Atrioventricular Connection
Double-Inlet Left Ventricle
DILV is the most common form of single ventricle described in all series. It represents 78% of the cases reviewed by Van Praagh et al. (1). The Van Praagh classification also distinguished four subgroups of DILV based on the great artery relationships: I, normally related great arteries; II, right anterior aorta; III, left anterior aorta; and IV, left posterior aorta (inverted). By combining the criteria distinguishing the types of DILV, three clinically observed forms occur (A-I, A-II, and A-III). In each of these clinical forms, other common associations include subaortic obstruction, pulmonary outflow tract obstruction, and conduction abnormalities (6).
Subaortic obstruction usually occurs in the setting of discordant VA connections and is located primarily at the VSD (bulboventricular foramen type). It also can be secondary to severe ventricular hypertrophy of muscle bundles within the hypoplastic right
ventricle (Fig. 50.7). Subaortic obstruction occurs more often in patients with associated atresia of the right AV valve and in patients who have had previous pulmonary artery banding procedures. Pulmonary artery banding can set the stage for progressive ventricular hypertrophy and obstruction in patients who have naturally occurring mild restriction at the VSD. These patients often present in infancy with associated coarctation of the aorta.
ventricle (Fig. 50.7). Subaortic obstruction occurs more often in patients with associated atresia of the right AV valve and in patients who have had previous pulmonary artery banding procedures. Pulmonary artery banding can set the stage for progressive ventricular hypertrophy and obstruction in patients who have naturally occurring mild restriction at the VSD. These patients often present in infancy with associated coarctation of the aorta.
Pulmonary outflow tract obstruction frequently occurs in DILV and may occur with either concordant or discordant VA connections. When subpulmonary obstruction occurs within the left ventricular chamber, it is most frequently due to posterior deviation of the infundibular septum. Alternatively, it may be due to anomalous attachments of the right AV valve or herniation of valvular tissue into the pulmonary outflow tract. Severe pulmonary valve stenosis and annular hypoplasia can occur. The valve is often bicuspid and thickened.
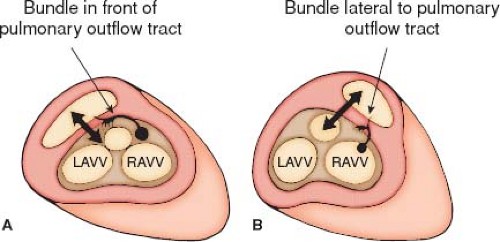
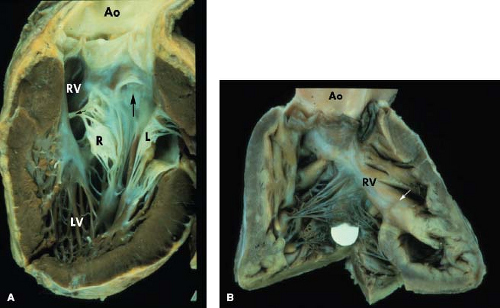
Conduction abnormalities similar to those described with discordant AV and VA connections (congenitally corrected transposition of the great arteries) can be present. When the AV connection is double inlet, the connecting AV node always is located anterolaterally at the acute margin of the right AV valve orifice (7,8). It subsequently perforates the annulus of the right AV valve to enter the main left ventricular chamber (Fig. 50.8). The nonbranching bundle courses along the right-sided rim of the VSD (outlet foramen) to reach the trabecular septum. The subsequent course of the bundle depends on the location of the rudimentary right ventricular chamber relative to the main ventricular chamber (MVC). If the hypoplastic right ventricle is right sided, the right margin of the VSD is adjacent to the anterolateral node, and the nonbranching bundle passes down directly onto the right rim of the VSD. The nonbranching bundle then descends toward the crest of the trabecular septum along the left ventricular aspect of the ventricular septum and branches beneath the septal crest. When the hypoplastic right ventricle is left sided, the right rim of the VSD, which is now also the anterior margin of the defect, is separated from the anterolateral node by the posterior great artery. The nonbranching bundle therefore must run a more extensive course anterior to the posterior semilunar valve annulus to reach the trabecular septum (Fig. 50.8). In all instances, it is located on the left ventricular aspect of the trabecular septum and descends down the right rim of the ventricular septum, branching below the septal crest. Although the nonbranching bundle always maintains the same relationship to the right rim of the VSD, it appears to be in a different location with right- or left-sided hypoplastic right ventricle when viewed from the hypoplastic right ventricle. If the hypoplastic right ventricle is left sided, the conduction tissue appears to run above the ventricular defect when viewed from the MVC. If the hypoplastic right ventricular chamber is right sided, the conduction bundle appears to run beneath the VSD when viewed from the MVC.
DILV with Normally Related Great Arteries (A-I Single Ventricle, “Holmes Heart”)
This form of DILV remains relatively rare, observed in only 15% of the Van Praagh series (Fig. 50.9) (7). Embryologically, the VSD providing communication between the LV and the hypoplastic right ventricle represents the primitive bulboventricular foramen. Frequently, the VSD creates significant subpulmonary obstruction, resulting in a somewhat balanced circulation with some hypoxia and low pulmonary artery pressure.
DILV with Right-Sided Hypoplastic Right Ventricle and Discordant VA Connections (A-II Single Ventricle)
DILV with right-sided hypoplastic subaortic right ventricle shares many anatomic features with a similar anomaly consisting of complete transposition of the great arteries with severe override and straddling of the right AV valve into the morphologic LV and associated hypoplasia of the morphologic right ventricle (Fig. 50.10). It
was observed in 25% of the cases of single ventricle reviewed by Van Praagh et al. (9). The embryologic development of various types of DILV representing the extreme forms of AV valve straddling (Fig. 50.5) easily supports the common features shared by these defects. In some cases, the AV valve morphology also may follow this schema, with the left-sided AV valve having morphologic features of a mitral valve and the right AV valve features of a tricuspid valve. With discordant VA connections, the aorta also typically is right anterior in location. Subaortic and pulmonary stenosis may occur. The conduction tissue enters the trabecular septum from an anterolateral node (7). The nonbranching bundle courses along the rightward rim of the VSD and subsequently distributes over the crest of the ventricular septum. Cook and Anderson reported an example of DILV with double-outlet LV and a small right-sided morphologic right ventricle which lacked both inlet and outlet components.
was observed in 25% of the cases of single ventricle reviewed by Van Praagh et al. (9). The embryologic development of various types of DILV representing the extreme forms of AV valve straddling (Fig. 50.5) easily supports the common features shared by these defects. In some cases, the AV valve morphology also may follow this schema, with the left-sided AV valve having morphologic features of a mitral valve and the right AV valve features of a tricuspid valve. With discordant VA connections, the aorta also typically is right anterior in location. Subaortic and pulmonary stenosis may occur. The conduction tissue enters the trabecular septum from an anterolateral node (7). The nonbranching bundle courses along the rightward rim of the VSD and subsequently distributes over the crest of the ventricular septum. Cook and Anderson reported an example of DILV with double-outlet LV and a small right-sided morphologic right ventricle which lacked both inlet and outlet components.
DILV with Left-Sided Hypoplastic Right Ventricle and Discordant VA Connections (A-III Single Ventricle)
DILV with a left-sided subaortic hypoplastic right ventricle is the most common type of univentricular connection observed in most reviews (9,10). It was encountered in 38% of the series review by Van Praagh et al. (9). In this early series, most examples of this defect were thought to represent examples of ventricular l-looping with the morphologic LV to the right and therefore with the morphologic mitral valve to the right of the morphologic tricuspid valve. Developmentally, one could speculate that this form of DILV shares anatomic features similar to AV and VA discordance (congenitally corrected transposition of the great arteries) but that severe overriding and straddling of the left-sided tricuspid valve ultimately resulted in predominant commitment of the left AV valve to the morphologic LV and DILV (Fig. 50.11). Thus, varying degrees of residual straddling of the left AV valve into the morphologic right ventricle would not be an unusual finding in this form of DILV. This seems a plausible and rational developmental explanation; however, in many instances of DILV, the AV valve anatomy is not sufficiently distinct to allow accurate differentiation of morphologic mitral or tricuspid valves. There also is significant variation in the size of the morphologic right ventricular cavity. It may be extremely hypoplastic—a tiny slit-like chamber—or can be 75% or 80% of the size of a normal right ventricular chamber, particularly when the left AV valve straddles into the right ventricle.
A variety of associated AV valve, semilunar valve, and outflow tract anomalies may occur. Subaortic obstruction is an important associated lesion that must be assessed when considering a modified
Fontan procedure. Significant ventricular hypertrophy substantially increases operative risk for Fontan operation because of associated ventricular diastolic abnormalities and elevated left ventricular end-diastolic pressure.
Fontan procedure. Significant ventricular hypertrophy substantially increases operative risk for Fontan operation because of associated ventricular diastolic abnormalities and elevated left ventricular end-diastolic pressure.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree