Balloon aortic valvuloplasty (BAV) is the primary therapy for congenital aortic stenosis (AS). Few reports describe long-term outcomes. In this study, a retrospective single-institution review was performed of patients who underwent BAV for congenital AS. The following end points were evaluated: moderate or severe aortic insufficiency (AI) by echocardiography, aortic valve replacement, repeat BAV, surgical aortic valvotomy, and transplantation or death. From 1985 to 2009, 272 patients who underwent BAV at ages 1 day to 30.5 years were followed for 5.8 ± 6.7 years. Transplantation or death occurred in 24 patients (9%) and was associated with depressed baseline left ventricular shortening fraction (LVSF) (p = 0.04). Aortic valve replacement occurred in 42 patients (15%) at a median of 3.5 years (interquartile range 75 days to 5.9 years) after BAV and was associated with post-BAV gradient ≥25 mm Hg (p = 0.02), the presence of post-BAV AI (p = 0.03), and below-average baseline LVSF (p = 0.04). AI was found in 83 patients (31%) at a median of 4.8 years (interquartile range 1.4 to 8.7) and was inversely related to post-BAV gradient ≥25 mm Hg (p <0.04). AI was associated with depressed baseline LVSF (p = 0.02). Repeat valvuloplasty (balloon or surgical) occurred in 37 patients (15%) at a median of 0.51 years (interquartile range 0.10 to 5.15) and was associated with neonatal BAV (p <0.01), post-BAV gradient ≥25 mm Hg (p = 0.03), and depressed baseline LVSF (p = 0.05). In conclusion, BAV confers long-term benefits to most patients with congenital AS. Neonates, patients with post-BAV gradients ≥25 mm Hg, and patients with lower baseline LVSF experienced worse outcomes.
Percutaneous balloon aortic valvuloplasty (BAV) is the mainstay of therapy for congenital aortic stenosis (AS), with low procedural mortality. Previously published long-term studies have described high rates of reinterventions after BAV. Aortic valve morphology and the diameter of the annulus have been associated with procedural success after BAV. Although baseline left ventricular (LV) shortening fraction (LVSF) has been associated with poor outcomes after surgical valvotomy for congenital AS in neonates, it has not been extensively studied in this setting. We reviewed our institutional experience with BAV for congenital AS over a period of 25 years. Our aim was to determine the incidence of significant aortic insufficiency (AI), repeat aortic valvuloplasty, aortic valve replacement (AVR), heart transplantation, and death. Additionally, we sought to determine the risk factors associated with these end points.
Methods
We queried our institutional database and hospital billing records to identify all patients who underwent BAV from 1984 through 2009. Patients were excluded if they ultimately underwent single-ventricle palliation. Demographic data, including age at catheterization, gender, weight, and associated cardiac lesions, were recorded. Outcomes after BAV were reviewed. The Social Security Death Index was queried to identify all deaths.
The baseline echocardiographic study was defined as the most recent study before BAV. Data retrieved from the echocardiographic report included aortic annular diameter and z score, aortic valve peak instantaneous and mean Doppler gradients, LVSF, and initial degree of AI (none, mild, moderate, and severe). AI was categorized according to the echocardiography laboratory standard, which conforms to currently available standards as defined by the American Society of Echocardiography. Significant AI was defined as moderate or severe AI. LV systolic function was assessed using the reported LVSF. Because of the variation in the normal range of LVSF among patients of varying ages and body surface areas, baseline LVSF z scores were used. Below-average baseline LVSF was defined as pre-BAV LVSF z score <0. Depressed LVSF was defined as pre-BAV LVSF z score <−2. LVSF z scores were based on available normative data. The final echocardiographic study was defined as the last study performed before repeat valvuloplasty, AVR, heart transplantation, death, or the end of the study. Data obtained from the final echocardiographic study included degree of AI, LVSF, and peak instantaneous and mean aortic valve gradients.
Procedural data were noted and recorded, including pre- and post-BAV catheter-derived peak-to-peak valve gradient, angiography-determined aortic valve annular diameter, the ratio of maximum balloon diameter to aortic valve annular diameter ratio (BAR), the number of separate balloon sizes used, and the use of a double-balloon technique. The degree of AI immediately before and after BAV, graded angiographically from none to severe, was noted.
Data are expressed as numbers and percentages for categorical variables and as mean ± SD for continuous variables. Data that did not fit a normal distribution are expressed as medians and interquartile ranges (IQRs). Significant AI was defined as moderate or severe AI on the final echocardiographic study before any clinical end point or termination of the study. The following clinical end points were studied: repeat valvuloplasty (repeat BAV, surgical aortic valvuloplasty, or surgical aortic valve repair), AVR, heart transplantation, and death. Continuous variables were compared using paired Student’s t tests, and categorical variables were compared using chi-square tests. Univariate time-dependent analysis was performed with log-rank analysis of Kaplan-Meier curves. Variables found to be significant on univariate analysis were included in the multivariate Cox regression analysis. Only 1 assessment of LVSF (LVSF z score, below-average LVSF, or depressed LVSF) was entered into a multivariate model at a time. Results of Cox regression analysis are expressed as hazard ratios with 95% confidence intervals. A p value ≤0.05 was deemed statistically significant. All data analysis was performed using SPSS version 17.0 (SPSS, Inc., Chicago, Illinois). Approval was obtained by the institutional research board.
Results
From 1984 to 2009, 307 patients underwent BAV at our institution. Catheterization reports were not available for 20 patients, and 15 patients ultimately underwent single-ventricle palliation. Thus, a total of 272 patients were included in our study. Patients ranged in age at BAV from 0 days to 30.5 years, with a median age of 3.0 years; 116 patients (42.6%) underwent BAV at age <1 year, including 50 patients (18.4%) who underwent BAV as neonates (age ≤30 days). Surgical valvuloplasty was performed before BAV in 24 patients (8.8%). Pre-BAV echocardiographic reports were available for review in 209 patients (77%). Before BAV, the mean and peak instantaneous Doppler aortic valve gradients on echocardiography were 46.5 ± 14.1 and 85.1 ± 25.0 mm Hg, respectively (n = 209). The average aortic valve annular diameter z score was −0.015 ± 2.0 (n = 203). The baseline LVSF was 40.1 ± 10.6%, and the baseline LVSF z score was 0.95 ± 5.0 (n = 159). Below-average baseline LVSFs were present in 50 of 159 patients (31%), of whom 25 (50%) were neonates. Depressed baseline LVSFs were present in 38 of 159 patients (24%), of whom 18 (47%) were neonates. Additional left-sided obstructive lesions were present in 78 patients (29%). The median follow-up time after BAV was 3.2 years, and a maximum of 26.1 years; 68 patients (25%) had follow-up >10 years.
At the time of catheterization, the average peak-to-peak gradient from the left ventricle to the ascending aorta was 63 ± 19 mm Hg, which was reduced to an average of 22 ± 13 mm Hg (p <0.01). The average decrease in aortic valve gradient was 42 ±17 mm Hg. A significant residual gradient immediately after BAV (≥25 mm Hg) was present in 76 patients (29%). Immediately after BAV, significant AI was present in 16 of 247 patients (6.5%). The BAR was 0.99 ± 0.12 (range 0.7 to 1.4). Angiographically moderate AI was seen in 4 of 247 patients (1.6%) immediately before BAV and in 16 of 247 patients (6.5%) immediately after BAV.
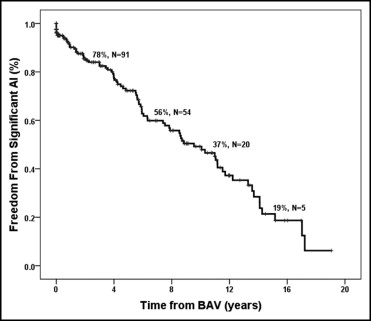
Of 247 patients in whom follow-up echocardiographic reports were available, 83 (34%) had significant AI at a median of 4.8 years (IQR 1.4 to 8.7) after BAV. Freedom from significant AI was 49% at 10 years after BAV ( Figure 1 ). The results of Cox regression analysis are listed in Table 1 . Factors associated with the development of significant AI included below-average baseline LVSF (p = 0.04) and depressed baseline LVSF (p = 0.02). Post-BAV gradient ≥25 mm Hg was negatively associated with the development of significant AI (p = 0.04). The development of AI was not influenced by neonatal BAV, the BAR, the aortic valve annular diameter z score, or the initial aortic valve gradient.
| Risk Factor | Hazard Ratio (95% Confidence Interval) | p Value |
|---|---|---|
| Aortic insufficiency | ||
| Neonatal BAV | 1.3 (0.59–2.8) | 0.53 |
| BAR | 7.4 (0.63–88) | 0.11 |
| Below-average baseline LVSF | 2.4 (1.1–5.5) | 0.04 |
| Depressed baseline LVSF | 2.5 (1.1–5.6) | 0.02 |
| Post-BAV gradient ≥25 mm Hg | 0.24 (0.06–0.90) | 0.04 |
| Repeat valvuloplasty | ||
| Neonatal BAV | 7.5 (2.0–29) | <0.01 |
| Depressed baseline LVSF | 3.5 (1.1–12) | 0.05 |
| Post-BAV gradient ≥25 mm Hg | 5.4 (1.2–24) | 0.03 |
| AVR | ||
| Additional left-sided obstructive lesion | 2.4 (0.60–9.4) | 0.22 |
| Below-average baseline LVSF | 4.7 (1.1–20) | 0.04 |
| Post-BAV gradient ≥25 mm Hg | 5.1 (1.3–20) | 0.02 |
| Death or heart transplantation | ||
| Neonatal BAV | 1.4 (0.31–6.1) | 0.68 |
| Depressed baseline LVSF | 4.7 (1.1–22) | 0.04 |
| Post-BAV gradient ≥25 mm Hg | 2.5 (0.48–12.7) | 0.28 |
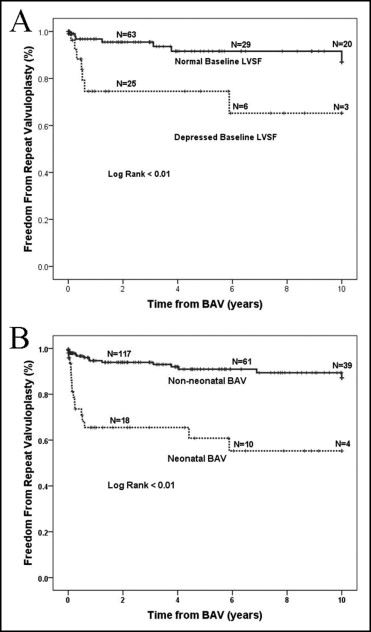
A second valvuloplasty was performed in 37 patients (14%) at a median of 0.51 years (IQR 0.10 to 5.15) after the initial BAV. Repeat BAV was performed in 23 patients, and surgical valvuloplasty was performed in 18 patients. A third valvuloplasty was performed in 4 patients (1.5%). Freedom from repeat valvuloplasty was 85% at 5 years, 83% at 10 years, and 65% at 15 years after BAV.
Multivariate Cox regression analysis demonstrated that neonatal BAV (p <0.01) and depressed baseline LVSF (p = 0.05) were associated with repeat valvuloplasty ( Table 1 ; Figure 2 ). Of the 50 patients who were neonates at the time of initial BAV, 17 (34%) underwent repeat valvuloplasty, compared to 20 of 222 non-neonates (9.0%) (p <0.01; Figure 2 ).
AVR was performed in 42 of 272 patients (15%) at a median of 3.5 years (IQR 75 days to 5.9 years) after BAV. Of the patients who underwent AVR, 30 (71%) had significant AI before AVR. The indication reported for AVR was isolated AI in 14 (33%), isolated AS in 10 (24%), combined AS and AI in 16 (37%), and AS with LV dysfunction in 2 (4.7%). Freedom from AVR was 84.2% at 5 years, 70.2% at 10 years, and 60.9% at 15 years after BAV ( Figure 3 ).




