8

Tuberculosis and Other
Mycobacterial Infections of the Lung
Tuberculosis
Epidemiology
History, High-Risk Groups, and Predisposing Conditions
Tuberculosis has been found among humans for millennia, with the lesions of skeletal tuberculosis (Pott’s disease) having been identified by molecular methods in both Egyptian and pre-Columbian Peruvian mummified and skeletal remains.1,2 Tuberculosis did not reach epidemic proportions, however, until the industrial revolution, which forced economically poor humans into close contact, an ideal situation for the transmission of tuberculosis.3 Prior to the epidemic spread of HIV, the annual incidence of newly diagnosed active tuberculosis was steadily declining in many developed countries, aided by multidrug, often “directly observed,” therapy.4 There was an increase in the number of reported cases of tuberculosis in the United States from 1985 to 1992.5 This is attributable to changes in the means of federal funding for tuberculosis control; the HIV/AIDS epidemic; the immigration from countries with a high prevalence rate of the disease; environmental factors, such as overcrowding and poverty; and the emergence of multidrug-resistant strains.4–12 This trend was reversed from 1992 through 2002, due to better enforcement of good public health measures, including directly observed, appropriate therapy and the treatment of HIV infection. Table 8–1 highlights the factors and predisposing conditions in the United States and Canada.
The majority of the tuberculosis in the world today exists in impoverished or developing countries. Twenty countries contain more than 80% of the world’s tuberculosis cases, including India, China, countries in Africa and Southeast Asia, and the republics of the former Soviet Union.5 The global tuberculosis fact sheet is astounding, with numbers that are difficult to comprehend. The World Health Organization (WHO) took an unprecedented step in 1993 and declared tuberculosis a global emergency. The recent fact sheet (No. 104) provided by WHO (http://www.who.int/mediacentre/factsheets/who104/en/) reports the following findings:
1. One third of the world’s population is currently infected with Mycobacterium tuberculosis.
2. A new infection with tuberculosis occurs every second, resulting in new tuberculosis infections in 1% of the world population annually.
3. Tuberculosis kills about 2 million persons per year, and the epidemic is growing.
4. HIV is significantly contributing to the spread of tuberculosis.
5. Newly acquired infections between 2002 and 2020 are projected at 1 billion persons. Of these, 150 million will get sick, and 36 million will die of tuberculosis.
The magnitude of the problem is indeed sobering.
Mycobacteriology
Tuberculosis is a disease of humans and certain animals caused by species of the M. tuberculosis complex,13 including M. tuberculosis, M. bovis, M. africanum, and M. microti. The Bacille Calmette-Guérin (BCG) vaccine is derived from M. bovis.13 M. tuberculosis is the most virulent of the mycobacteria, and is an obligate pathogen. The only other Mycobacterium species that shares this characteristic is M. leprae. The nontuberculous mycobacteria (NTM), also called atypical mycobacteria or mycobacteria other than tuberculosis (MOTT), are found in the environment, cause a variety of infections, and are discussed in greater detail later in this chapter.
HIV infection |
Age <6 years, adolescents, elderly |
Close contact with infected person |
Immigration from endemic country |
Aboriginal peoples |
Homeless |
Low socioeconomic status |
Overcrowded housing |
Recent tuberculin skin test conversion |
Inactive tuberculosis |
Non-HIV immunosuppression |
Iatrogenic |
Other concomitant diseases |
Silicosis |
Some malignancies (head and neck, lung) |
Diabetes mellitus (unstable or poorly controlled) |
Hemodialysis |
End-stage renal disease and uremia |
Gastrectomy and ileojejunal bypass |
The initial laboratory studies used to establish the diagnosis of tuberculosis consist of direct acid-fast staining of sputum or other respiratory specimens, and culture. If the patient cannot produce sputum, an early-morning gastric aspirate or bronchoalveolar lavage (BAL) may be used. All isolates of M. tuberculosis should be submitted for susceptibility testing, because resistance, even multidrug resistance, is common.6,14 Therefore, it is the obligation of the clinician, surgeon, and pathologist to ensure that tissue specimens are adequate for both histology and culture. New molecular techniques offer hope of rapid diagnosis.
Basic Pathology
The spectrum of histopathologic patterns of tuberculosis have been extensively described.15–28 Primary tuberculosis, describes the cellular and tissue response to M. tuberculosis in a host that lacks acquired immunity to the same or antigenically related mycobacteria. The primary infection is usually in the lung. A systemic immune response follows, whereby the host acquires immunity and sensitivity.29 In animal models, the implantation of tubercle bacilli in the alveoli of a previously uninfected host initiates an acute exudative, nonspecific, acute (neutrophilic) inflammatory reaction.16,27 Macrophages and polymorphonuclear leukocytes then enter the alveoli and phagocytose bacilli. The bacilli multiply within the macrophages. The development of resistance at this stage may cause the infection to abort without the development of acquired immunity and tuberculin hypersensitivity.25,30
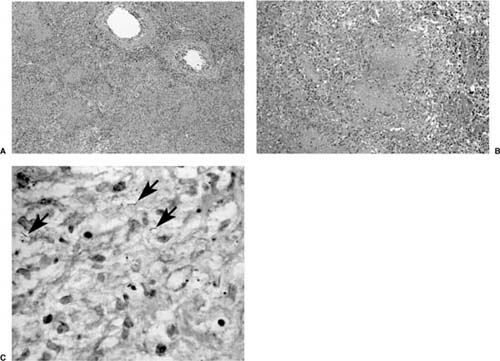
The interaction of the macrophages and T lymphocytes over the next 3 to 8 weeks leads to the emergence of acquired immunity, delayed hypersensitivity (positive tuberculin skin test), and ultimately the development of classic necrotizing and/or nonnecrotizing granulomas. Accelerated phagocytosis and intracellular killing mark this phase, as does the migration of activated monocytes to the site, which transform into tissue histiocytes and cluster to form granulomas. Some coalesce to form multinucleated giant cells or Langhans cells. Although largely absent in the early stages, lymphocytes are recruited to the lesions at this time, correlating with the development of tuberculin sensitivity. These lymphocytes, which encircle the epithelioid cells, are composed primarily of T-suppressor cells.31 The appearance of characteristic “caseous” (cheesy) necrosis in the center of the granulomas (Fig. 8–1) also parallels the onset of delayed hypersensitivity.24,32
The histologic hallmark of tuberculosis is the granuloma, either caseous (a term that describes the gross appearance of a or necrotizing tuberculous granuloma) or noncaseous (nonnecrotizing). Although rarely shadows of residual lung structures, such as blood vessels, bronchioles, and alveolar septa, may be detectable in the necrotic center of these granulomas, it is far more likely that the necrotic center is homogeneous (Fig. 8–2).33 The classic tuberculoid granuloma, also known as the “hard” tubercle of Rich, occurs in patients with unipmaired immunity. Histologic findings that correlate most closely with culture- or stain-positive cases of tuberculosis are the presence of necrotizing or nonnecrotizing granulomas. Organisms are less frequently associated with a purely neutrophilic response (but may be) and are not seen in tissues where only chronic inflammation and fibrosis are present.34 Nonspecific findings that may also be present include edema, hemorrhage, and an accumulation of detached pneumocytes and histocytes in the alveoli.33 Rarely histiocytes or even small granulomas may be present within vessel walls, but significant vascular necrosis is not a feature of tuberculosis. Regional lymph nodes are almost invariably involved, and contain granulomas as well as nonspecific reactive features. The nodal involvement may be more prominent than the parenchymal lesion in some instances.
Mycobacteria are demonstrated using the Ziehl-Neelsen acid-fast stain (Fig. 8–2C), or the more sensitive auramine-rhodamine fluorescent stain. The organisms are most readily identified near the interface of a zone of necrosis and viable tissue, due to higher oxygen levels and pH here in comparison to the avascular center of the granuloma. Examination of sections from two blocks containing necrotizing granulomas is usually sufficient to identify acid-fast bacillus (AFB), although occasionally more sections may be needed to find very rare organisms. The organisms in the Ziehl-Neelsen–stained sections are thin, bright red, and typically beaded. They may appear slightly curved. In addition, mycobacteria often lie side by side, associated with one another. The organisms are gram-positive and often have a beaded appearance in sections stained with tissue modifications of Gram’s stain.
FIGURE 8–1 Upper lobe nodule of reactivation tuberculosis with central cavitation and characteristic caseation.
Patients with advanced disease or profound immunodeficiency can have a variety of reaction patterns, including those described above. In acute caseating miliary tuberculosis (also called nonreactive tuberculosis) there is a minimal cellular reaction, a paucity of activated macrophages with few or no epithelioid cells, tissue necrosis, and abundant AFB. This is equivalent to the “soft” tubercle of Rich.24,35,36 Typically there is no cavitation or fibrosis. This is an uncommon reaction in progressive primary or postprimary disease, and is usually associated with hematogenous dissemination that may affect any organ. It is often fatal.35 The difference has been emphasized between this condition and the usual form of miliary tuberculosis (discussed later), in which paucibacillary epithelioid granulomas typify the histology. The latter form of miliary spread may be acute or chronic. Chronic or insidious tuberculosis may be clinically misdiagnosed, especially in the elderly. When either of these forms of tuberculosis fails to display clinical or radiographic evidence of disease, it has been termed “cryptic disseminated tuberculosis.”37
FIGURE 8–2 (A,B) Necrotizing granulomatous inflammation due to M. tuberculosis. Early cavitation can be seen in some of them (A). (C) The Ziehl-Neelsen stain shows rare organisms.
The destruction of infected tissues is a consequence of tuberculosis. The degree of damage to functioning tissue may be underestimated by macroscopic and radiographic evidence of scarring, but may be more clearly delineated with physiologic studies, such as a ventilation-perfusion scan. Fibrosis and dystropic calcification follow the resorption of caseous material, and may in turn be occasionally followed by dystrophic ossification.38 Microscopic calcification in children may occur in less than 2 months, as witnessed by the Lubeck disaster in 1930, in which a vaccine containing virulent tuber-cle bacilli was given in error to children instead of BCG.39 Visualization of calcification by x-ray requires at least a year or longer. Resorption of calcium from the lung and lymph node lesions occurs subsequently in about one third of children with a primary complex over the next number of years.40
The presence of calcification denotes that repair has occurred but does not imply an inactive or sterile lesion.39 Although bacilli in healed primary complex lesions usually die, AFBs have been reported to be present in stained histologic sections in one half the calcified primary lesions.19,24 In the preantibiotic era, tubercle bacilli were reportedly cultivated from up to 20% of calcified primary lesions at postmortem examination.41 Thus, the presence of what appears to be a calcified primary tuberculous lesion on chest x-ray should not be taken as confirmation of inactive disease. The shift in the morbidity rate to the elderly, reflecting the persistence of remotely acquired organisms, is supportive of the idea that calcified lesions may harbor viable tubercle bacilli. Nevertheless, it has been found that the majority of fibrosed and calcified lesions at autopsy or resection contain no culturable bacilli.23,42
Primary tuberculosis: no preexisting immunity and hypersensitivity |
Exogenous first infection: never infected; includes congenital tuberculosis |
Exogenous reinfection: a new infection after loss of immunity and hypersensitivity due to: |
Waning immunity over time and death of tubercle bacilli |
Acquired immunodeficiency |
“Senescence” of immune function |
Progressive primary tuberculosis (possibly inadequate acquired immunity/inadequate “natural resistance” |
Postprimary tuberculosis, also known as reinfection, secondary, and adult tuberculosis; occurs in persons with preexisting immunity and hypersensitivity |
Endogenous reactivation of remote, latent, or inactive tuberculous disease. |
Exogenous infection of the Bacille Calmette-Guérin (BCG) vaccinated |
Exogenous superinfection of a person who was previously infected and with (usually) inactive disease |
Classification of Tuberculosis
The natural history of an infection by M. tuberculosis is influenced by a variety of factors that include the virulence of the infecting organism, the infecting dose, and natural and acquired host factors that confer resistance to infection. Table 8–2 shows the clinical classification or tuberculosis.
Tuberculosis may be categorized as primary or postprimary disease. A person’s initial infection by M. tuberculosis is primary tuberculosis. The primary disease may progress, a disease aptly termed progressive primary tuberculosis, and may take on the features of adult or postprimary disease, which includes apical cavitary disease. Both endogenous recrudescence and exogenous reinfection occur in patients who have been infected in the past, and also result in the postprimary pattern of tuberculosis.43 Primary and postprimary tuberculosis, if not contained by the immune response, may disseminate hematogenously throughout the lungs and multiple other organs, a condition termed miliary tuberculosis.19,44–46
Transmission and Acquisition of Infection
Tuberculosis is a communicable disease spread primarily through inhalation.43,47,48 Less commonly, it is acquired through ingestion or dermal implantation. The lung is the portal of entry for most infections, and pulmonary tuberculosis is the predominant form of the disease.4 Close inspection of patients with extrapulmonary tuberculosis often reveals infection in the lung. Infections caused by M. bovis are usually acquired by ingestion, with the primary manifestations of disease in the alimentary tract. Any of the members of the M. tuberculosis complex may be implanted accidentally (e.g., a laboratory or surgical accident), wherein the primary manifestation would most commonly be in the skin.
The mycobacteria are transmitted via droplet nuclei, which are 2 to 10 μm in diameter. The small size of these infectious droplet nuclei enable them to remain suspended in the air (i.e., they resist settling) and reach the terminal bronchioles and alveoli. Animals studies suggest that tuberculosis may be transmitted through the inhalation of a single bacillus.47,49 Although this may be true, epidemiologic and experimental evidence underline the importance of the infecting dose.49–51
M. tuberculosis is an obligate aerobe, which in part is responsible for the localization of disease in the more apical portions of the lung.52,53
Primary Tuberculosis
Congenital and Perinatal Tuberculosis
Delayed and missed diagnoses are frequent.54–57 Tubercle bacilli from an infected mother may pass through the placenta to the fetus. Miliary tuberculosis may result from placental seeding. Infection may enter the fetus through the umbilical veins, which causes primary lesions in the liver with tuberculous lymph nodes in the porta hepatis. Lesions may also occur in the lungs, suggesting further passage of the bacilli through the ductus venosus. Tuberculous involvement of the lungs and hilar and mediastinal lymph nodes, without associated hepatic lesions, indicates aspiration of infected amniotic fluid or inhalation of tubercle bacilli or birth canal secretions.
Congenital tuberculosis is characterized by a nonimmune, nonreactive response, with abundant caseation and an unusually large number of bacilli.55 There may be multiple infectious foci in the primary site of infection or miliary distribution. Polymorphonuclear leukocytes predominate; lymphocytes, epithelioid histiocytes, and Langhans giant cells are rare.
Childhood Acquisition
Lobar and segmental infections of the lung may occur when a young child contracts tuberculosis, and the characteristic presentation is associated with the small diameter and compressibility of the airways (Fig. 8–3). Historically, these lesions have been given a variety of terms, including the rather awkward term epituberculosis.58 This variety of terms arose to contrast the apparent extent of the lung involvement, the relatively benign course, and the relative well-being of the child, in many cases. All of this suggested erroneously that the disease was not true tuberculosis. The term epituberculosis, however, is best supplanted by the term segmental lesion.50,59
FIGURE 8–3 Primary tuberculosis complicated by “epituberculosis” in an infant closely exposed to active tuberculosis. Tuberculin conversion over 2 months. Sequence of radiographs showed right and left hilar and right paratracheal lymphadenopathy. Consolidation with mild volume loss occurred in the anterior segment of the right upper lobe. Minimal scarring remained in this segment after 3 years’ optimal antimicrobial therapy.
The pathology consists of extrinsic bronchial obstruction from enlarged lymph nodes and intrinsic obstruction from edema and granulomas. Fistula formation with drainage of adjacent caseous lymph nodes into contiguous bronchi may also be present. The lobe or segments that are subtended by the obstruction may be the seat of obstructive hyperinflation, atelectasis, secondary nontuberculous bacterial pneumonia, one or more isolated granulomas, or aspiration of caseous material.27
Atelectasis most commonly affects the anterior segments of the upper lobes and the right middle lobe, and in the preantibiotic era full reexpansion of the affected segment was uncommon.44,50,59 Residual scarring was common in the absence of antibiotic therapy, as was residual bronchostenosis or bronchiectasis.59 The latter may mimic reactivated tuberculosis when a secondary infection occurs in these bronchi in later life, particularly when this affects the anterior segment of the upper lobe. Calcified residua of the primary complex, parenchymal, or lymph node lesions may occasionally erode into a bronchus forming a broncholith, which may be expectorated or cause bronchial obstruction, leading to distal infection and lung abscess.60,61
Primary disease that is severe or neglected may result in enlarged hilar or mediastinal lymph nodes that cause impaired venous return and superior vena cava syndrome, tracheal obstruction at the thoracic inlet, rupture into the mediastinum, erosion into a blood vessel or pericardium, compression of the left recurrent laryngeal or phrenic nerve, or compression of or erosion into the esophagus, leading to esophageal diverticulum and bronchoesophageal fistula.62
Primary Acquisition in Adolescents and Adults
Primary infection in adults may be subclinical.19,20,24,44,46,63 Bronchial obstruction and dissemination are less common in adults compared with children, likely because the bronchial tree and the immune system are fully developed. Endobronchial tuberculosis is seen with post-primary disease in adults and is thought to result from the implantation of the tubercle bacilli on adjacent bronchial mucosa, but this pathogenesis remains speculative.
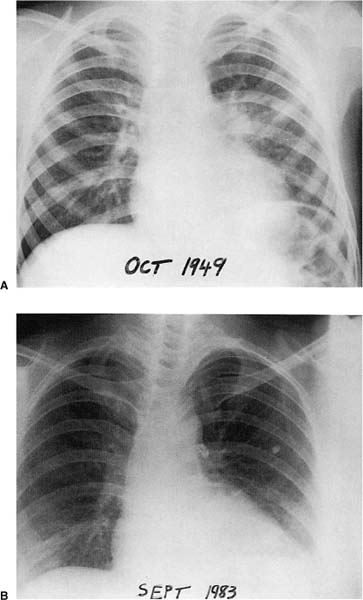
The course of primary tuberculosis is generally benign, particularly since the advent of chemotherapy. The majority of people infected with virulent tubercle bacilli are unaware of the infection, except for a delayed hypersensitivity reaction to tuberculin on skin testing. The typical uncomplicated primary lesion is the combination of a small (usually less than 1 cm in diameter), often in-apparent parenchymal lesion in the upper aspect of a lower lobe or lower aspect of an upper lobe, coupled with enlarged, ipsilateral hilar, and, less commonly, paratracheal nodes (Fig. 8–4)—the Ghon complex (named for the Prague pathologist Anton Ghon, who described it in 191264). Bilateral adenopathy is uncommon except with left-sided primary foci.65,66 Massive lymphadenopathy may occur in patients with poor nutrition, general health, or socioeconomic conditions.60,67 A minority of patients may demonstrate radiographic evidence of a residual hyalinized scar or calcification at the site of the primary (Ghon) lesion, in the lung parenchyma, and in the hilar or paratracheal lymph nodes. This combination is referred to as the healed primary complex.64
Progressive Primary Tuberculosis
Progressive primary tuberculosis occurs following primary infection in a minority of patients. Progressive primary tuberculosis may occur as an extension of the primary focus, as a tuberculous bronchopneumonia, or may develop the features of typical postprimary tuberculosis. Cavitation and progressive primary disease are more likely in infancy and around puberty, and in the elderly.19,68 Cavitation in children is uncommon, except in persons lacking innate resistance.44
FIGURE 8–4 Primary tuberculosis. (A) Primary focus in the left lung with enlarged ipsilateral hilar glands at age 6 years. (B) Typical calcified primary complex at age 40 years in the same patient.
Postprimary (Adult-Type) Tuberculosis
This form of the disease occurs in persons previously infected with M. tuberculosis who have acquired immunity, including hypersensitivity to tuberculoprotein. Exogenous reinfection may play a more frequent role in the causation of postprimary disease in countries with high infection rates.42,69 Subsequent infection following previous BCG vaccination can result in a postprimary pattern of disease.70
The vigorous cell-mediated immune response that results from the renewed multiplication of tubercle bacilli in postprimary disease leads to prominent liquefactive necrosis and cavitation characteristic features of this stage of tuberculosis. The histopathology reflects the pathophysiology, which is directed at containment of the infection and healing by fibrosis, a process that is greatly accelerated by antituberculous therapy.71 The tubercle bacilli may spread by direct extension, by the formation of satellite tubercles, by the bloodstream, by the lymphatics (although this is not prominent in post-primary disease), and through the airways; liquefied caseous material expelled into the airways may result in tuberculous bronchopneumonia, often in a contralateral lower lobe.
Prior to advent of drug therapy (chemotherapy), the behavior and the healing of tuberculous cavities was the cause of much discussion, because the persistence of a cavity correlated with imperfect healing and a potential for the transmission of infection. Collapse therapy was used at that time to promote closed healing, with inspissation of the contents or scar formation following obliteration of the communicating bronchus. It was observed that even without effective chemotherapy there could rarely be healing of persistently open cavities, which subsequently became lined with bronchial or squamous epithelium.71 More commonly, such open healed cavities would have a fibrous lining. Modern treatment commonly allows for the rapid closure of cavities, frequently leaving little evidence of their previous existence radiographically.
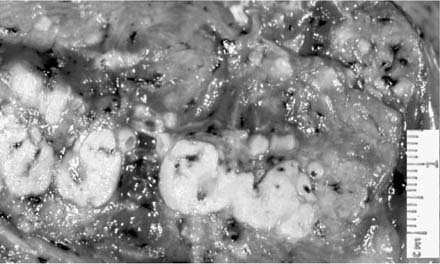
The characteristic gross appearance of postprimary tuberculosis is caseous necrosis, frequently with cavitation, a variable amount of fibrosis, and retraction of the lung parenchyma (Fig. 8–5). In contrast to primary tuberculosis, lymph node involvement is minimal. Fraser and colleagues44 reviewed the anatomic distribution of tuberculous lesions in the lung. For reasons discussed earlier, the apical and posterior segments of the upper lobes are predominantly involved.72 These authors recognized the following patterns, one or more of which can be seen in any individual case: (1) local exudative lesions; (2) local fibroproductive lesions, sometimes with calcification; (3) cavitation; (4) bronchogenic spread with acute tuberculous bronchopneumonia; (5) miliary disease; (6) bronchiectasis; (7) bronchostenosis; (8) tuberculoma; and (9) tuberculous pleurisy and empyema.
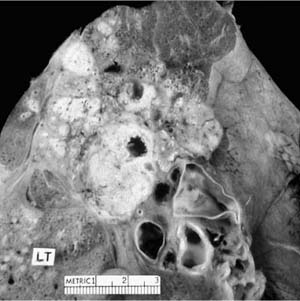
FIGURE 8–5 Left upper lobe postprimary tuberculosis with cavitation and surrounding fibrosis.
As a consequence of healing, fibrosis and cicatrization commonly extend between the upper pole of the hilum and the apex and tend to elevate the hilum on that side. This also causes a loss of volume in the affected lobe. The same fibrosis tends to pull the upper mediastinum toward the side of the lesion, sometimes distorting the trachea quite sharply. These types of complications are uncommon in patients with adequate health care, but may still be encountered in patients living in impoverished areas or in situations where the disease has been neglected and allowed to become chronic and progressive.
Tuberculomas are commonly surrounded by so-called satellite lesions.44 Histologically, the pattern is variable with caseous necrosis and extensive fibrosis. Caseating and noncaseating granulomas, giant cells, and lymphocytes are present together with fibroblasts and fibrosis. Following treatment, AFBs may be scanty but can usually be found.
Bronchiectasis following postprimary tuberculosis usually occurs in the upper lobe, as a sequel of the parenchymal disease in the apical and posterior segments, and thus may be relatively asymptomatic. Bronchiectasis and bronchostenosis predispose to secondary infection, hemoptysis, and atelectasis.
Extension to the pleura is common (discussed later). Contiguous spread to the pericardium is rare, except in patients co-infected with HIV, and is often associated with pleuritis.73 Lymphatic spread to the pericardium may be a more common pathway.73 Contiguous spread from the lung, lymph node, vertebra, pericardium, or pleura may also involve the aorta, causing focal aneurysmal dilatation and rupture.73
Massive hemoptysis may result from tracheal, bronchial, or parenchymal tuberculous lesions. Cavitary disease is usually the cause of parenchymal bleeding. Dilatation of small to medium-size arteries that pass tangentially across the fibrous capsule of the cavity may be found (Rasmussen’s aneurysm), or a saprophytic fungus ball may occupy a tuberculous cavity (discussed later).71
Laryngeal tuberculosis is a complication that has become rare in the United States. However, it was not an uncommon consequence of chronically active, advanced, cavitary disease in the past, and resulted from implantation of bacilli. Before the use of antimicrobial therapy, laryngeal tuberculosis was reported to occur in up to one third of those dying from pulmonary tuberculosis.74 Laryngeal disease correlated with the extent of the pulmonary tuberculosis and correlated well with the occurrence of tuberculous enterocolitis.75
Extensive destruction of the lung may follow neglected pulmonary tuberculosis. Posttuberculous lung destruction has been reported to have occurred in 11% of 1600 cases.76 The same report denotes a tendency for this to affect the left lung much more commonly than the right. The suggested reasons for this predilection included the fact that the left main bronchus was longer and narrower than its counterpart on the right side, and the peribronchial space was narrowed by the closely adjacent aorta, thus tending to obstruction when adjacent lymph nodes enlarged.77 Furthermore, a series of hemodynamic changes tended to occur by which perfusion was impaired, which encouraged progression of tuberculosis once the disease became established.
In adults, mediastinal tuberculous lymph nodes may rarely cause a bronchoesophageal fistula, paralysis of the phrenic nerve, and superior vena caval syndrome.44 Remnants of surgical collapse procedures occasionally may still be encountered, including plombage, thoracoplasty, phrenic nerve paralysis, as well as bronchopleural fistulas and distortions of bronchopulmonary anatomy and drainage, from combinations of resection and tuberculous disease.
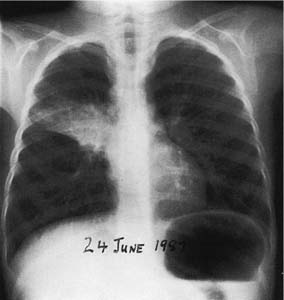
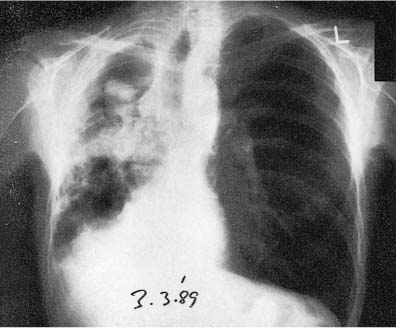
A fungus ball (most often due to aspergillus) may complicate a tuberculous cavity.78–81 This commonly results in hemoptysis, which may be fatal78,81 (Fig. 8–6). Seventeen percent of 544 healed tuberculous cavities were stated to have developed aspergillomas over a period of 3 years in a British survey.82 Some aspergillomas undergo spontaneous resolution, but most persist. The condition is associated with high mortality, often from the associated pulmonary disease. Thickening of the overlying pleura, which may be considerable, is frequent in the presence of an aspergilloma and may antedate the visualization of the fungus ball by 2 to 3 years.79,83
Fungal balls caused by members of the Mucorales order and by Pseudallescheria boydii have also been reported in patients with tuberculous cavities.78 Other fungi that may colonize or invade a tuberculous cavity include Candida, Nocardia, Sporothrix, Penicillium species, and Coccidioides immitis.78 It is necessary in the differential diagnosis of an intracavitary mass to also consider an intracavitary blood clot, hamartoma or other neoplasm, and pulmonary gangrene, which can also give an air-crescent sign.78,84 Other causes of a similar appearance include an echinococcal cyst, a dermoid cyst, and Rasmussen’s aneurysm (in a tuberculous cavity).85 A solitary report records the simulation of an intracavitary mass due to the invagination of a loculated pleural effusion into an adjacent cavity.86 In addition, it is important to remember that a residual tuberculous cavity or bronchiectasis may also become colonized by nontuberculous mycobacteria (discussed later).
FIGURE 8–6 Posteroanterior (PA) chest radiograph of a 45-year-old male diabetic and alcoholic showing an aspergilloma in one of several tuberculosis cavities. Dense pleural reaction with scarring and contraction of the right lung has caused ipsilateral displacement of the mediastinum and trachea and elevation of the right diaphragm. Exposure to a sister with tuberculosis 20 years before, with subsequent strong positive tuberculin reaction and small scar in right upper lobe, preceded endogenous reactivation of tuberculosis.
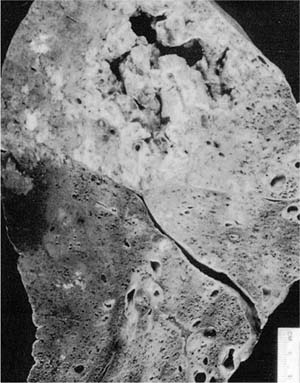
Pulmonary gangrene has been described as a rare complication of advanced or acute tuberculous pneumonia (Fig. 8–7), the recognition of which is important because it is frequently fatal if unrecognized and untreated.87 Tuberculous arteritis, followed by thrombosis, leading to ischemic necrosis, appears to be the pathogenic sequence that results in a ragged, mobile, slough or sphacelus, which occupies a cavity formed by the coalescence of radiolucent necrotic zones in an area of tuberculous pneumonic infiltrates.
Factors Affecting Localization of Primary and Postprimary Disease
The distribution of the inhaled droplet nuclei is determined by the pattern of ventilation and the volumes of the various lung lobes and, therefore, tends to emphasize the middle and lower lung zones, although any lobe may be the site of initial infection.21 A single subpleural lesion occurs in the majority of cases, although in about 15 to 25% of patients there are multiple primary loci.21,88,89 Lymphohematogenous dissemination follows the local tuberculous pneumonitis that develops. The tubercle bacilli may then be harbored in favored locations, the most important of which is the apical aspects of the lungs. The vertebral bodies, kidneys, lymph nodes, meninges, and the epiphyses of long bones are other favored locations.
FIGURE 8–7 Gross photograph showing tuberculous gangrene of the lung.
The localization of postprimary tuberculosis is often apical,72 which has been suggested to be related to the relatively higher oxygen tension in this region. Although tubercle bacilli are strictly aerobic and would benefit from such an environment, it is also possible that the benefit is indirect and secondary to the unfavorable effect the higher oxygen tension has on the macrophage, thereby permitting intracellular growth.52 The localization of progressive primary disease may also be influenced by this factor, being common in the apical and posterior segments of the upper lobe.44 The protective effect of mitral stenosis is of interest, because this is associated with higher pulmonary arterial pressure and increased apical arterial blood flow; the converse applies to pulmonary stenosis.53 High vascularity and increased oxygen tension may also play a role in harboring the tubercle bacillus in the extrapulmonary favored sites of retention, such as the ends of long bones, in vertebrae, and in the renal cortex following hematogenous dissemination.
Another concept has been studied and is favored by some regarding the retention of M. tuberculosis, nontuberculous mycobacteria, and Histoplasma capsulatum in the upper lobes of the lungs. This concept suggests that the combination of lowered blood flow, and consequently lowered lymph flow, and reduced respiratory movement at the apex results in reduced lymphatic drainage and less effective removal of antigen and pathogens from these locations.53,90
Other Presentations of Tuberculosis of the Lungs
The acute respiratory distress syndrome may occur in association with either miliary or advanced tuberculosis.91–93 In addition, an acute, nonnecrotic, polymorphonuclear pneumonia with numerous bacilli has been reported, as a terminal event in pulmonary tuberculosis in which there has been loss of tuberculin sensitivity.26
Silicosis
Occupational lung disease caused by the inhalation of dust containing silicon dioxide (usually quartz) has been known since the 18th century to be associated with tuberculosis.94,95 Dust control measures, improvements in working conditions, and advances in drug therapy for tuberculosis have considerably reduced the prevalence of silicosis and silicosis-associated tuberculosis (silicotuberculosis).94 Although the incidence is currently less than in the past, there remains an association between an exposure to silica and an increase in active tuberculosis, which is greater than for individuals who lack similar occupational exposures.94,96 In one study in northern Canada, one third of miners with silicosis had sputum specimens positive for AFB at some time during their lives.97
Silica, like some immunosuppressive agents, impairs macrophage function and facilitates intracellular replication of mycobacteria and early release from the cell.98 Endocytosis of silica by alveolar macrophages causes intracellular lysosomal rupture, which leads to cell death and release of macrophage products, which, in turn, promotes inflammation.99
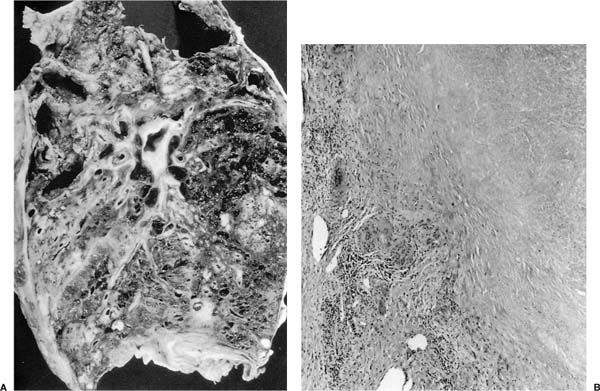
The radiographic changes secondary to established silicosis may obscure those of superimposed tuberculosis. Sudden changes in size of the lesion, cavitation, new opacities, especially in the apical regions, or the development of pleurisy should raise the suspicion of concurrent active tuberculosis, particularly in a tuberculin reactor. The presence of complicating tuberculosis, however, may not be recognized until autopsy, but even then its detection may be difficult (Fig. 8–8).95 The microscopic appearance of the tuberculous lesion may be altered, with a reduction or absence of epithelioid cells and Langhans giant cells, as well as a loss of the lymphocyte collar.100 Necrosis in the center of a silicotic nodule almost always means that concomitant tuberculosis is present and confirmation sought by performing acid-fast stains.
The role of M. tuberculosis in exciting the formation of progressive fibrosis is still debated.95 The presence of tuberculosis, however, does not appear necessary for the development of massive fibrosis in patients with silicosis.94 The decline in the prevalence of both these diseases in developed countries will determine the future epidemiologic relationship between these two conditions. Better evidence for deciding this debate may come from a more thorough knowledge of the immunology and the genetics associated with silicosis.101,102
FIGURE 8–8 Tuberculosis complicating silicosis in a 76-year-old man. (A) Gross radiograph of an apical cavitary lesion and multiple nodules. (B) Microscopically the granulomas and silicotic nodules merge. The necrosis and giant cell reaction at the edge of this silicotic nodule indicate this nodule is infected. Note absence of lymphocyte collar around this “granuloma.” Rare acid-fast organisms were present at the edge.
Extrapulmonary Tuberculosis
M. tuberculosis may spread throughout the body by three means: endobronchial spread, contiguous spread, and lymphohematogenous dissemination.
There are several possible manifestations of extrapulmonary tuberculous. Cervical adenitis may be an early manifestation of extrapulmonary disease, which results from the earlier lymphohematogenous spread of the organism, or may appear from within a few months to years following the initial infection. Skeletal tuberculosis may appear promptly or many years later. Genitourinary tuberculosis characteristically is a late occurrence after many years.
Pleural Tuberculosis
A tuberculous pleural effusion occurs in association with primary in up to 10% of recently infected persons50 (Fig. 8–6). It is uncommon in younger children. Tuberculous effusions that follow primary infections often resolve, but these patients are at high risk for developing chronic tuberculosis within 5 years.88,103 A tuberculous pleural effusion may also occur many years after primary infection as a complication of postprimary tuberculosis or as an isolated effusion. Primary effusions usually occur unilaterally, and on the same side as the primary complex (more commonly the right side), supporting the hypothesis that the pleural effusion arises from the extension of a subpleural caseous tuberculous focus or lymph node that has extended into the pleural space.104 Bilateral effusions or those occurring on the contralateral side of the primary complex should raise the possibility of miliary tuberculosis or HIV infection.50,88
In the era prior to specific antimycobacterial therapy, it was noted that the vast majority of effusions in patients with primary pleural effusions secondary to tuberculosis resolved. However, when the effusion developed in adolescence and young adulthood, it was associated with a significant risk for the development of postprimary tuberculosis within 5 to 10 years.88 This risk was between ~7% for children59 and 62% for young adults.103
Pleural and subpleural granulomas can be seen on both visceral and parietal surfaces in primary tuberculous effusions. These follow the lymphatics on the visceral surface and are discrete and, therefore, susceptible to sampling error on needle biopsy.105 Focal or diffuse fibrosis may be a long-term sequela.
This is in distinct contrast to a tuberculous empyema, which results from a bronchopleural fistula that occurs when a large caseous or cavitary lesion ruptures into the pleural space. This is seen most commonly in postprimary tuberculosis. Examination of the pleural fluid reveals almost pure pus. The sequelae of the inflammatory response may include fibrous obliteration of the costophrenic angle, occasionally more extensive fibrosis and calcification, and sometimes a calcified pleural plaque. A trapped lung or a pneumothorax may be occur, which may require surgical intervention.44 The advent of modern antimicrobial agents has made such complications rare in countries where therapy is readily available, but examples of chronic empyema that have persisted for long periods of time and still yield viable bacilli have been reported.106 A tuberculous empyema was almost uniformly fatal prior to the introduction of antituberculous drugs.
The release of mycobacterial antigens into the pleural space evoke a cell-mediated hypersensitivity reaction, the main mechanism dictating the volume and cellular nature of the effusion.107 The immunopathogenesis of this reaction, as well as the diagnostic and prognostic considerations stemming from it, have been reviewed.88,107 AFBs are rarely detected in a direct smear of the pleural fluid in classic primary tuberculous pleural effusion.107–109 Culture of pleural fluid is positive in only 25 to 60% of cases. A pleural biopsy may be required to establish the diagnosis. Histology of the pleural biopsy demonstrates typical granulomatous pleuritis in about 60 to 80% of cases, but AFBs are identified in only 5 to 18% of such cases. Culture of pleural tissue confirms the presence of M. tuberculosis in 55 to 76% of cases. Not surprisingly, the assessment of multiple specimens increases the diagnostic yield. The combination of acid-fast staining, culture of both fluid and tissue, and histology of pleural tissue confirm the diagnosis in more than 95% of patients, which reiterates the complementary nature of anatomic pathology and clinical microbiology.107,108
The differential cell content of a tuberculous pleural effusion is quite variable. The leukocyte concentration is usually less than 5008 cells/μL. The cellular composition of pleural fluid consists of lymphocytes in two thirds of the cases. The proportion of lymphocytes may be as high as 85 to 90%.108 In one series, however, the pleural fluid of 38% of such patients had a predominance of neutrophils. There may be occasional atypical mesothelial cells, which may suggest malignancy, but mesothelials are generally sparse in such effusions.110 The pleural fluid generally has a protein of >2.5 g/dL, a pH ≤7.3, and a glucose that is diminished compared with the serum concentration, but rarely >20 mg/dL. Less than 10% of the effusions are serosanguinous.
Elevations in the adenosine deaminase (ADA) level in pleural fluid has been proposed as a test with reasonably good sensitivity and specificity; this assay is a simple and relatively inexpensive test, which is most important because resources are often limited where tuberculosis is most prevalent.111 Several other pathologic conditions, such as parapneumonic effusions and effusions caused by lymphoproliferative disorders, have pleural fluid findings that overlap with those seen with tuberculosis and must be considered in the differential diagnosis.112 It has been proposed that pleural fluid ADA activity greater than 60 U/L combined with a predominately lymphocytic differential cell count strongly are suggestive of tuberculosis.113 ADA activity higher than 40 U/L, however, does not exclude parapneumonic effusions or effusions caused by lymphoproliferative disorders, but does differentiate 90% of the other malignant effusions, including mesothelioma, and also pulmonary embolism.113
Interest continues in biochemical markers that are predictive of tuberculosis, such as ADA, interferon-γ (IFN-γ), lysozyme, and β2-microglobulin.108,111,114 However, recent advances in both the sensitivity and specificity of mycobacterial DNA detected by means of polymerase chain reaction (PCR), and more recently real-time PCR and other methods of homogeneous nucleic acid amplification and detection, will likely overtake the use of biochemical tests, at least in places where the technology is available, practicable, and affordable.109 One study, which examined pleural effusions by traditional PCR for the IS6110 sequence that is specific for M. tuberculosis, detected M. tuberculosis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree