Truncus Arteriosus
Allison K. Cabalka
William D. Edwards
Joseph A. Dearani
Persistent truncus arteriosus is an uncommon congenital cardiovascular malformation. There is no striking gender difference in frequency, although most series contained more male than female subjects. Truncus arteriosus usually occurs as an isolated cardiovascular malformation, although it has been reported in association with anomalies of other systems, particularly the DiGeorge or velocardiofacial syndrome (microdeletion chromosome 22q11.2) (1,2,3,4). Maternal diabetes has been implicated as a risk factor for truncus arteriosus. The anomaly has occurred in dizygotic twins (5) and siblings, and there is an increased incidence of cardiac malformations in relatives of children with this lesion (6,7,8). Because corrective operation for this malformation was first performed more than 30 years ago (9) ever-increasing numbers of postoperative patients are now reaching adolescence and adulthood. Patients who have had truncus arteriosus corrected need continued follow-up care throughout life. During the last 30 years, surgical correction of truncus arteriosus during infancy has become routine (10,11).
Embryology
The embryonic truncus arteriosus lies between the conus cordis proximally and the aortic sac and aortic arch system distally. Partitioning of the truncus arteriosus, which is intimately associated with conal and aortopulmonary septation, was reviewed by Van Mierop et al. (12) and more recently by Bartelings and Gittenberger-de Groot (13). Truncus swellings, similar in appearance to endocardial cushions, divide the truncal lumen into two channels: the proximal ascending aorta and the pulmonary trunk. As the proximal portion of this truncal septum fuses with the developing conal septum (derived from conal swellings), the right ventricular origin of the pulmonary trunk and the left ventricular origin of the aorta are established. Valve swellings develop from truncal tissue at this line of fusion, and the excavation of these swellings leads to formation of the aortic and pulmonary valves in their respective sinuses. Along the aortic sac, the paired sixth aortic arches (primitive pulmonary arteries) migrate leftward, and the paired fourth aortic arches shift rightward. Invagination of the aortic sac roof thereby forms an aortopulmonary septum that eventually fuses with the distal extent of the truncal septum. Accordingly, the right and left pulmonary arteries originate from the pulmonary trunk, and the aortic arch emanates from the ascending aorta. The spiral course of the truncoaortic partition produces the normal intertwinement of the great arteries.
When conotruncal or truncoaortic septation does not proceed normally, various congenital ventriculoarterial anomalies may result (12). One of these anomalies is truncus arteriosus, in which a single arterial trunk exits from the heart. Also, either deficiency or absence of the conal (infundibular) septum produces a large ventricular septal defect. Because the conal septum also contributes to the development of the anterior tricuspid leaflet and the medial tricuspid papillary muscle, these structures may be malformed. The single truncal valve may be deformed and functionally insufficient or, less commonly, stenotic (14). If vestiges of distal truncoaortic septation develop, the pulmonary arteries may arise together from a short pulmonary trunk; otherwise, they arise separately from the truncal root.
Pathology
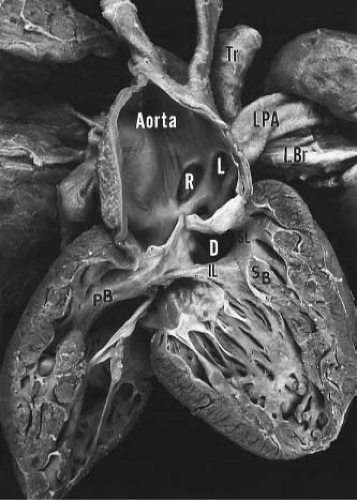
Truncus arteriosus is characterized by a single arterial vessel that arises from the base of the heart and gives rise to the coronary, pulmonary, and systemic arteries (Fig. 42.1). Origin of the pulmonary arteries from this single artery serves to differentiate truncus arteriosus from pulmonary valve atresia, a condition in which a single arterial vessel also receives the entire output of both ventricles
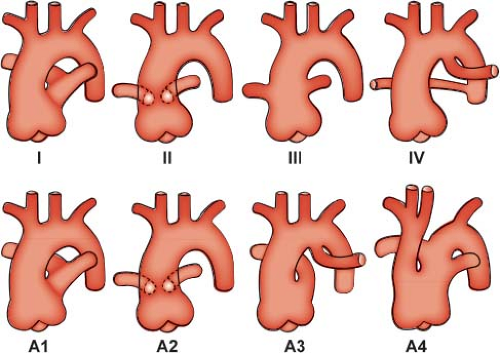
but in which the pulmonary arteries do not arise directly from the ascending portion of this single great artery. Collett and Edwards (15) recognized four types of truncus arteriosus on the basis of the anatomic origin of the pulmonary arteries. In type I, a short pulmonary trunk originating from the truncus arteriosus gives rise to both pulmonary arteries. When both pulmonary arteries separate from the truncus arteriosus, with no vestige of a main pulmonary artery (MPA), they may arise close to one another (type II) or at some distance from one another (type III). The type IV truncus arteriosus is now considered to represent a form of pulmonary atresia with ventricular septal defect and is not discussed further in this chapter.
but in which the pulmonary arteries do not arise directly from the ascending portion of this single great artery. Collett and Edwards (15) recognized four types of truncus arteriosus on the basis of the anatomic origin of the pulmonary arteries. In type I, a short pulmonary trunk originating from the truncus arteriosus gives rise to both pulmonary arteries. When both pulmonary arteries separate from the truncus arteriosus, with no vestige of a main pulmonary artery (MPA), they may arise close to one another (type II) or at some distance from one another (type III). The type IV truncus arteriosus is now considered to represent a form of pulmonary atresia with ventricular septal defect and is not discussed further in this chapter.
Van Praagh and Van Praagh (16) have proposed an expanded classification system that also includes two commonly associated abnormalities of the great arteries. Their type A1 corresponds to type I of Collett and Edwards, and type A2 encompasses types II and III (Fig. 42.2). Type A3 includes cases with absence of truncal origin of one pulmonary artery, with blood supply to that lung from the ductus arteriosus or from a collateral artery. Last, type A4 is associated with underdevelopment of the aortic arch, including tubular hypoplasia, discrete coarctation, or complete interruption.
The ventricular septal defect in truncus arteriosus is generally large and results from either absence or pronounced deficiency of the infundibular septum. The defect is cradled between the two limbs of the septal band and is roofed by the truncal valve cusps (see Fig. 42.1). In most instances, fusion of the inferior limb and the parietal band causes muscular discontinuity between the tricuspid valve and the truncal valve (15). Accordingly, the membranous septum is intact, and the defect is of the infundibular type. When such fusion fails to occur, tricuspid–truncal valvular continuity is present, and the defect (which now involves the membranous septum) is of combined membranous and infundibular types. Rarely, the ventricular septal defect in truncus arteriosus may be small and restrictive or even absent (17).
Among 400 cases of truncus arteriosus from four publications reviewed by Fuglestad et al. (14), the truncal valve was tricuspid in 277 (69%), quadricuspid in 86 (22%), bicuspid in 35 (9%), pentacuspid in 1 (0.3%), and unicommissural in 1 (0.3%). The semilunar valve is in fibrous continuity with the mitral valve in all patients but is continuous with the tricuspid valve in only a minority. By overriding the ventricular septum, the truncus arteriosus has a biventricular origin in 68% to 83% of patients (15,18). In 11% to 29% of patients, the truncal valve arises entirely from the right ventricle, whereas in 4% to 6% of patients, it emanates entirely from the left ventricle.
The anatomic cause for truncal valve insufficiency is variable and includes thickened and nodular dysplastic cusps, prolapse of unsupported cusps or of conjoined cusps with only a shallow raphe, inequality of cusp size, minor commissural abnormalities, and annular dilation (14,19). Truncal valve stenosis, when present, usually is associated with nodular and dysplastic cusps (19). The truncal root is frequently dilated, and the truncal sinuses are often poorly developed.
A right aortic arch with mirror-image brachiocephalic branching, occurring in 21% to 36% of patients (19,20), is associated more commonly with truncus arteriosus than with any other congenital cardiac malformation except pulmonary atresia with ventricular septal defect. Rarely, a double aortic arch persists. Hypoplasia of the arch, either with or without coarctation of the aorta, occurs in 3% of patients (18). Interrupted aortic arch occurs relatively frequently (11% to 19% of patients) (19,21) and is accompanied by ductal continuity of the descending thoracic aorta. It is frequently associated with the DiGeorge syndrome.
The ductus arteriosus is absent in approximately half of the patients with truncus arteriosus, but it remains patent postnatally in nearly two-thirds of patients in whom it is present. The relative sizes of the aorta and the ductus arteriosus tend to vary inversely, such that the ductus arteriosus is particularly large in patients with underdevelopment of the aortic arch (type A4 truncus).
The pulmonary arteries most commonly arise from the left posterolateral aspect of the truncus arteriosus, a small distance above the truncal valve. Type I truncus arteriosus is observed in 48% to 68% of patients, type II in 29% to 48% (18), and type III in 6% to 10% (15,18). In type II, the left pulmonary artery ostium generally is somewhat higher than that of the right pulmonary artery. Rarely, in the setting of interrupted aortic arch, this ostium may arise to the right of the right pulmonary artery ostium and cause crossing of the pulmonary arteries posterior to the truncus arteriosus (19).
Stenosis of the pulmonary artery ostia or arteries is uncommon. In rare instances, deformed truncal valvular tissue may obstruct the pulmonary ostia during ventricular systole. In general, however, unless pulmonary arterial banding is performed, the pulmonary vascular bed will be exposed to systemic arterial pressure.
In truncus arteriosus, one pulmonary artery may be absent. Of the Mayo Clinic’s previously published series of patients with truncus arteriosus, 16% (11 of 70) had only a single pulmonary artery (22). In 9 of the 11 patients, the pulmonary artery was absent on the side of the aortic arch. Thus, in truncus arteriosus, the pulmonary artery most frequently is absent on the side of the aortic arch, in contrast to tetralogy of Fallot, in which the pulmonary artery more frequently is absent on the side opposite the aortic arch.
This chapter does not consider either the so-called pseudotruncus arteriosus, which is actually a form of pulmonary valve atresia with ventricular septal defect, or “hemitruncus,” in which one pulmonary artery arises from the ascending aorta and the other emanates from the right ventricle and clearly has a well-developed pulmonary valve at its origin. The embryologic basis for these deformities appears to be different from that for true persistent truncus arteriosus.
Knowledge of variations in coronary arterial origin and distribution, which are common in truncus arteriosus, is important to the surgeon. Because the left anterior descending coronary artery frequently is relatively small and displaced leftward, the conus branch of the right coronary artery, in a compensatory manner, is usually prominent and supplies several large branches to the right ventricular outflow tract (23,24). The posterior descending coronary artery arises from the left circumflex artery (left coronary dominance) in 27% of patients with truncus arteriosus (25), which is about three times the frequency of this variation in the normal population. Anomalies of coronary ostial origin, involving 37% to 49% of patients with truncus arteriosus (25), are common, regardless of the number of truncal valve cusps. In general, however, the left coronary artery tends to arise from the left posterolateral truncal surface and the right coronary artery from the right anterolateral surface (18,25).
In the setting of a single coronary ostium, frequently associated with left coronary dominance, all three major epicardial branches originate from this common site, or the right coronary artery may be absent (19). When two ostia exist, both may arise from the same truncal sinus; one may take origin from the expected site of the noncoronary sinus, or both may arise normally. High ostial origin, above the truncal sinotubular junction, occurs often, but when the origin is at or slightly above a truncal valve commissure, the involved ostium (most commonly the left) may be slit-like and functionally stenotic. Conceivably, dysplastic valvular tissue also could obstruct an otherwise normal coronary ostium. Rarely, the left coronary artery originates from the pulmonary trunk (18,26). Combinations of the aforementioned coronary anomalies are frequently observed.
The location of the conduction tissue in truncus arteriosus is also of surgical importance. The sinus node and the atrioventricular node are normal in location and structure. The atrioventricular bundle courses to the left of the central fibrous body, and the left bundle branch emanates along the left ventricular septal subendocardium, just beneath the membranous septum (27). The right bundle branch travels within the myocardium of the ventricular septal summit, attaining a subendocardial course at the level of the moderator band. In most instances in which the ventricular septal defect is truly infundibular and the membranous septum is intact, the atrioventricular conduction tissue is somewhat distant from the rim of the defect. In patients with combined membranous–infundibular ventricular septal defect, however, the conduction tissue passes along the left aspect of the posterior-inferior rim of the defect.
The anomalies most commonly associated with truncus arteriosus are right aortic arch, interrupted aortic arch, absent ductus arteriosus, patent ductus arteriosus, unilateral absence of a pulmonary artery, coronary ostial anomalies, and an incompetent truncal valve. A secundum atrial septal defect has been noted in 9% to 20% of patients, an aberrant subclavian artery in 4% to 10%, a persistent left superior vena cava draining into the coronary sinus in 4% to 9%, and mild tricuspid stenosis in 6% (18,20). Total or partial anomalous pulmonary venous connection in association with truncus arteriosus also has been described (22,28). Rare associated anomalies that have been reported include tricuspid atresia, mitral atresia, ventricular inversion, and association with the asplenia complex. We have encountered one patient with both truncus arteriosus and complete atrioventricular septal defect. Extracardiac anomalies, present in 21% to 30% of autopsy cases of truncus arteriosus, include skeletal deformities, hydroureter, bowel malrotation, and multiple complex anomalies.
Among the secondary complications of truncus arteriosus, biventricular hypertrophy is frequent, and dilation of ventricular chambers is prominent when truncal valve insufficiency exists. If there is massive cardiac hypertrophy, chronic subendocardial myocardial ischemia may develop (even with normal epicardial coronary arteries). As a result of chronic exposure of the pulmonary vasculature to systemic arterial pressure, hypertensive pulmonary vascular disease (plexogenic pulmonary arteriopathy) may develop. The arteriolar lesions often develop more rapidly and to a more severe extent in truncus arteriosus than in isolated ventricular septal defect. With chronic truncal valve insufficiency, pulmonary venous hypertension also may develop.
As patients with surgical repair survive into adulthood, progressive dilation of their aorta (original truncal artery) often occurs but is rarely associated with complications, including dissection or rupture (29).
Manifestations
Clinical Features
In most patients with truncus arteriosus, congenital heart disease is recognized during early infancy, often during the neonatal period. During the 1990s, intrauterine diagnosis became possible with fetal echocardiography (30). The clinical features depend largely on the volume of pulmonary blood flow and whether associated significant truncal valve insufficiency is present.
During the first weeks of life, persistence of increased pulmonary arteriolar resistance present during fetal life may cause mild cyanosis with little evidence of cardiac decompensation, unless severe truncal valve insufficiency also is present. As pulmonary resistance gradually decreases and flow through the lungs increases, the cyanosis may disappear. However, tachypnea, tachycardia, excessive sweating, poor feeding, and other signs of pulmonary overcirculation may appear. If truncal valve insufficiency is severe, the signs and symptoms of heart failure may appear shortly after birth. The additional volume load produced by this associated problem always adds to the increasing demands placed on the heart as pulmonary flow increases.
In the uncommon situation in which the infant has naturally occurring stenosis of the pulmonary arteries, cyanosis may be present at birth and may intensify with increasing age. However, such stenosis protects the child from pulmonary overcirculation that would otherwise occur with falling pulmonary resistance. Severe cyanosis, in addition to the signs of heart failure, may be present early if the child has both naturally occurring stenosis of the pulmonary artery and severe insufficiency of the truncal valve.
Physical Examination
Physical findings are related primarily to the volume of pulmonary blood flow and the presence or absence of truncal valve insufficiency. Patients with increased pulmonary blood flow have little or no cyanosis. The peripheral pulses are accentuated and may be bounding. The pulse pressure usually is increased owing to runoff into the pulmonary vascular bed during diastole and is
accentuated in the setting of truncal valve insufficiency. A left precordial bulge may be noted, and a systolic thrill often is palpable along the left sternal border. The heart usually is overactive. The first heart sound is normal and frequently followed by an ejection click, which echocardiographic studies have shown to coincide with maximal opening of the truncal valve. The second heart sound usually is loud and single. The occasional auscultatory or phonocardiographic observation of a split second sound in these patients with a single semilunar valve may be caused by delayed closure of some of the cusps of the abnormal truncal valve. An apical third heart sound often is present. A loud pansystolic murmur maximal at the lower left sternal border and radiating to the entire precordium is most often heard. An apical diastolic low-pitched murmur caused by increased flow across the normal mitral valve frequently is audible.
accentuated in the setting of truncal valve insufficiency. A left precordial bulge may be noted, and a systolic thrill often is palpable along the left sternal border. The heart usually is overactive. The first heart sound is normal and frequently followed by an ejection click, which echocardiographic studies have shown to coincide with maximal opening of the truncal valve. The second heart sound usually is loud and single. The occasional auscultatory or phonocardiographic observation of a split second sound in these patients with a single semilunar valve may be caused by delayed closure of some of the cusps of the abnormal truncal valve. An apical third heart sound often is present. A loud pansystolic murmur maximal at the lower left sternal border and radiating to the entire precordium is most often heard. An apical diastolic low-pitched murmur caused by increased flow across the normal mitral valve frequently is audible.
The patient with truncal valve insufficiency usually has an early diastolic high-pitched murmur that is heard best along the left sternal border. A truly continuous murmur is uncommon in truncus arteriosus and, when present, is usually suggestive of pulmonary artery ostial stenosis. Continuous murmurs are common in patients with pulmonary valve atresia/ventricular septal defect, where either a patent ductus arteriosus or systemic collateral arteries provide pulmonary blood flow. Because the differential diagnosis of truncus arteriosus includes this lesion, a continuous murmur is strongly suggestive of pulmonary atresia rather than of truncus arteriosus. Patients with heart failure may exhibit the additional signs of tachypnea, crepitant rales, hepatomegaly, and neck-vein distension.
Cyanosis is present, and clubbing of the fingers and toes may be seen in patients with decreased pulmonary blood flow caused by naturally occurring pulmonary artery stenosis, pulmonary artery banding, or pulmonary vascular disease. If there is no associated truncal valve insufficiency, the peripheral pulses and pulse pressure are nearly normal. The apical diastolic murmur often is not present. These patients are less likely to have signs and symptoms of cardiac decompensation.
Electrocardiographic Features
The electrocardiogram (ECG) usually shows a normal frontal plane QRS axis or minimal right-axis deviation. Generally, normal sinus rhythm is present, and the conduction times are not prolonged. Combined ventricular hypertrophy occurs frequently. Left ventricular forces are particularly prominent in patients with increased pulmonary blood flow. Left atrial enlargement also is common in this group. Patients with normal or decreased pulmonary flow may exhibit right ventricular hypertrophy only.
Radiologic Features
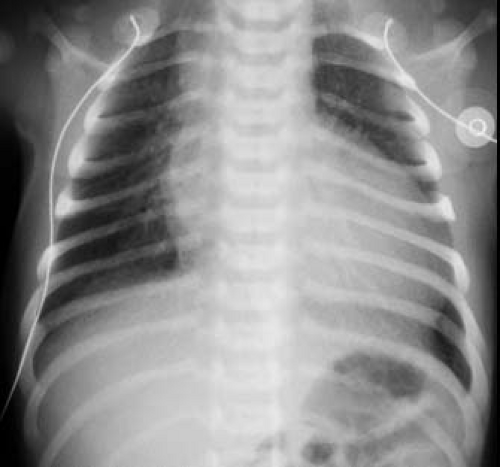
Typically, radiography of the chest shows moderate cardiomegaly and increased pulmonary vascular markings (Fig. 42.3). The aortic arch is right-sided in approximately one-third of patients, and the combination of a right aortic arch and increased pulmonary vascularity is strongly suggestive of truncus arteriosus. Type I truncus arteriosus frequently is associated with a relatively superiorly located proximal left pulmonary artery, which usually can be distinguished on a frontal chest radiograph. A dilated truncal root is common. Although the pulmonary vascular markings typically are increased, variation in the pulmonary vascular pattern can be seen. In truncus arteriosus with unilateral absent pulmonary artery, the pulmonary vascular markings are markedly diminished on the side without the pulmonary artery (usually the left side). In addition, pulmonary vascular obstructive disease is common in patients with truncus arteriosus and is reflected in the chest radiograph by disproportionate enlargement of the central pulmonary arteries associated with accentuated tapering of the distal pulmonary arterial tree.
Echocardiographic Features
The use of 2-D, Doppler, and color Doppler echocardiography has greatly increased the ability to determine accurately the cardiac anatomy and, in most cases, the hemodynamics in truncus arteriosus (31,32). Subcostal windows are used to document abdominal visceral situs and atrial situs, in addition to the position of the cardiac apex. A single great vessel arising from the heart typically is seen from a subcostal image (Fig. 42.4A), and assessment of the atrial septum is performed best from this location as well. Subcostal windows provide additional views for evaluation of the truncal valve function, truncal root, and pulmonary artery branch anatomy. The parasternal long-axis view demonstrates the deficiency in the ventricular septum and the overriding great artery, with continuity between the truncal valve and the mitral valve. Slightly higher position in the parasternal long-axis view can be used to visualize the origin of the pulmonary trunk or branches (Fig. 42.4B). Further imaging is required to document the presence of a single arterial trunk and lack of a pulmonary outflow tract from the ventricle. High parasternal short-axis views will provide visualization of the pulmonary arteries arising directly from the posterolateral aspect of the truncal root, typically bifurcating into the right and left pulmonary arteries. Persistence of a short MPA segment is seen in truncus arteriosus type I (Van Praagh type A1, Fig. 42.5). Separate origins of the pulmonary branches are seen in type II (type A2). When only one pulmonary artery is present, as in type III truncus (type A3), the remaining pulmonary artery origin must be documented, typically originating from the aortic arch or ductus arteriosus. The short-axis view is also useful in evaluating the anatomy of the truncal valve leaflets (number and morphology), as well as visualization of the coronary arteries and their origins, and the location and extension of the ventricular septal defect. Suprasternal notch imaging is critical for evaluation of the aortic arch anatomy, as interruption of the aortic arch may be associated with truncus arteriosus (type A4). Right-sided aortic arch
also is common in truncus arteriosus and can be determined from short-axis imaging of the arch branching pattern. In addition, the pulmonary artery branches also can be visualized from the suprasternal notch, excluding any important branch stenoses (Videos 42.1 to 42.14) (33).
also is common in truncus arteriosus and can be determined from short-axis imaging of the arch branching pattern. In addition, the pulmonary artery branches also can be visualized from the suprasternal notch, excluding any important branch stenoses (Videos 42.1 to 42.14) (33).
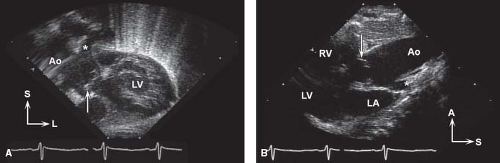 Figure 42.4 A: Subcostal coronal view with anterior angulation in newborn with truncus arteriosus type I and moderately severe truncal regurgitation. Arrow indicates doming, thickened truncal valve; aortic root (Ao) continues anteriorly/superiorly. Origin of the pulmonary trunk indicated by the asterisk. L, left; LV, left ventricle; S, superior. B: High parasternal long-axis view in same newborn with truncus arteriosus type I, with the doming truncal valve (arrow) and posterior origin of the pulmonary trunk (asterisk). A, anterior; LA, left atrium; LV, left ventricle; RV, right ventricle; S, superior.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|