Chapter 28 Truncus Arteriosus
Truncus arteriosus was recognized in 1798,1 and the clinical and necropsy findings were described in 1864.2 Humphreys summarized the reports up to 1932, and Lev and Saphir3 critically reviewed published accounts during the following decade. The malformation accounts for approximately 1% to 2% of cases of congenital heart disease at necropsy and approximately 0.7% to 1.2% of all congenital cardiac malformations.4
Truncus arteriosus is characterized by a single great artery with a single semilunar valve that leaves the base of the heart and gives rise to the coronary, pulmonary, and systemic circulations.4–7 A second semilunar valve is neither present nor implied. The single arterial trunk receives the output from both ventricles, so a ventricular septal defect is obligatory.4,5,8 Type IV, or pseudotruncus with a biventricular aorta and an atretic pulmonary valve, is now uniformly considered to be a form of pulmonary atresia with ventricular septal defect rather than truncus arteriosus (see Chapter 18).9 Edwards, with disarming candor, stated, “Twenty-eight years after the introduction of the term, we doubt that the condition which Collett and Edwards had called truncus Type IV exists.”5 Similarly, a solitary pulmonary trunk referred to in 1814 by Farrell10 is considered to be a variety of aortic atresia.11,12 Hemitruncus, a term no longer used, referred to a rare anomaly in which one pulmonary artery branch arose from the ascending aorta just above the aortic sinuses and in which the main pulmonary artery with the other branch arose in its normal position.13,14 Rarely, the main pulmonary artery arises anteriorly and proximally at the level of the sinus of the common arterial trunk.15
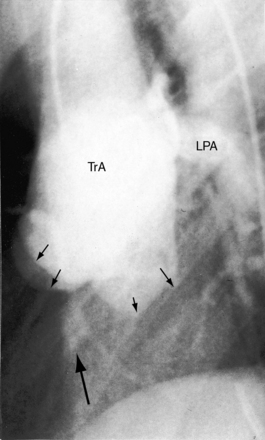
The truncus is large because it accepts the entire output of both systemic and pulmonary circulations (Figures 28-1 and 28-2A), although inherent medial abnormalities contribute to the dilation.16 Agenesis of the ductus arteriosus occurs in 50% to 75% of cases,4,7,17 which is not surprising because a fetal ductus is not needed to channel pulmonary arterial blood into the aorta.18
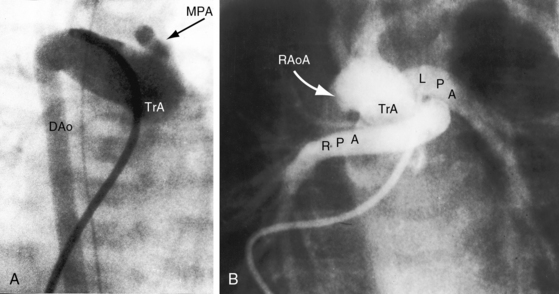
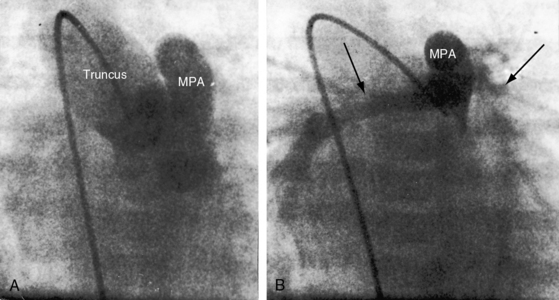
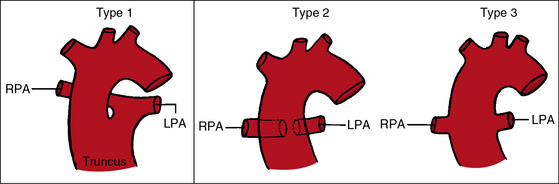
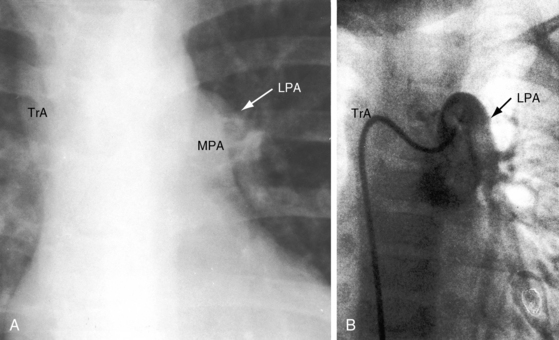
The greatest anatomic variability is in the branching patterns of the common trunk.19,20 In 1949, Collett and Edwards5 classified truncus arteriosus into four types based on the origins of the pulmonary arteries. The first three types were reconsidered by van Praagh and van Praagh7 in 1965 and form the basis of current terminology (Figure 28-3). Type 4 is now regarded as pulmonary atresia with ventricular septal defect (see previous and see Chapter 18). The most common variety is type 1, which is characterized by a short main pulmonary artery that originates from the truncus and gives rise to the right and left pulmonary arteries (Figures 28-2A, 28-4, and 28-5).7 Type 2 and type 3 of Collett and Edwards were originally defined by right and left pulmonary arteries that arose from separate ostia at either the side or the back of the truncus (see Figure 28-3).4,5 These two types are now grouped within a single category referred to as type 2 (see Figures 28-1B, and 28-3).7 In about 15% of cases, the right or left pulmonary artery is absent or hypoplastic (Figure 28-4B). An absent21 or hypoplastic pulmonary artery is usually concordant with the side of the aortic arch,22 which is right-sided in as many as 30% of patients with truncus arteriosus (see Figures 28-1 and 28-5).4,7,19,23 Rarely, there is a double aortic arch24,25 or an interrupted aortic arch.26
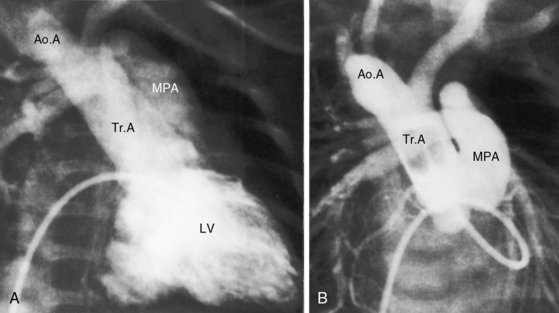
Figure 28-5 Angiocardiograms from a male neonate with truncus arteriosus type 1 and DiGeorge syndrome (see Figure 28-6). A, The left ventriculogram (LV) visualized the truncus (Tr.A) that continued as a right aortic arch (AoA). The main pulmonary artery (MPA) originated directly from the truncus. B, Contrast material into the truncus delineated a main pulmonary artery originating directly from the truncus and continuing as a right aortic arch (AoA).
In approximately two thirds of patients, the truncal valve has three leaflets, and its has either two leaflets (bicuspid) or four leaflets (quadricuspid) in most of the others. Very rarely, the valve is pentacuspid or hexacuspid (see Figures 28-10 and 28-22).19 A normal trileaflet aortic valve differs from a trileaflet truncal valve because of the presence of truncal raphes and cuspal inequality and because trileaflet truncal valves tend to be thickened and focally or diffusely dysplastic.17,27,28 A trileaflet truncal valve with raphes and cusps in excess of three represents a morphogenetic combination of aortic and pulmonary valves.7 Truncal valves are poorly supported therefore and are frequently incompetent.4,29 Stenotic truncal valves are less common,4,27,30,31 may be dysplastic,32 and may calcify in older adults.33
The coronary arteries in truncus arteriosus are defined by their relationship to the truncal sinuses and by their epicardial courses (see Figure 32-12).7,8,34–36 Coronary arterial ostia appear after division of the embryonic truncus is complete during normal morphogenesis.37 There are initially six coronary artery anlagen (primordia), three from the aorta and three from the pulmonary artery.38 Anlagen in the three pulmonary sinuses and in one of the aortic sinus normally undergo involution, leaving two coronary arteries that arise from two persistent anlagen in the right and left aortic sinuses. In truncus arteriosus, the sinus substrates to which coronary arteries are assigned are influenced by failure of septation of the embryonic truncus and by developmental abnormalities of the truncal valve.34,36 The left coronary artery tends to arise from the left posterior aspect of the truncus, and the right coronary artery tends to arise from the right anterior aspect of the truncus, whether the truncal valve is bicuspid, tricuspid, or quadricuspid (see Figure 32-12).8 When the truncal valve is quadricuspid, which is usually the case, coronary artery orifices originate in opposite sinuses rather than in adjacent sinuses, and high ostial origins are frequent.8,36 The right coronary artery is dominant in about 85% of cases; the conus branch of the right coronary artery is large, and the left anterior descending artery is small.8 There is an increased incidence of single coronary artery (see Chapter 32).34,36 An ostial membrane of the left coronary artery has been reported.39
A ventricular septal defect results from absence or deficiency of the infundibular septum and is almost always nonrestrictive and roofed by the truncal valve, setting the stage for inadequate support and truncal valve regurgitation.40 The biventricular truncal valve is assigned equally to the two ventricles, or predominantly to the right ventricle but only infrequently to the left ventricle.4,6,17 Cardiovascular anomalies commonly associated with truncus arteriosus include a right aortic arch (see previous and see Figures 28-1 and 28-5), truncal valve abnormalities (see previous and see Figures 28-10 and 28-22), anomalies of origin and distribution of the coronary arteries (see previous and see Figure 32-12), absence of the right or left pulmonary artery (see Figure 28-4B), and atresia of the ductus arteriosus. Abnormalities that occur sporadically include single ventricle, aberrant subclavian artery, left superior vena cava, and total anomalous pulmonary venous connection.41,42 When truncus arteriosus occurs with complete interruption of the aortic arch, the interruption is distal to the origin of the left carotid artery.17 The left subclavian artery arises from the descending aorta, and a patent ductus provides continuity from truncus to descending aorta.17
A common arterial trunk is a feature of normal early embryogenesis, an understanding of which sheds light on the morphogenesis of persistent truncus arteriosus.43,44 Septation of the arterial pole of the normal heart begins with the appearance of two opposing truncal cushions that rapidly enlarge and fuse to form the truncal septum. The proximal truncal septum normally fuses with the distal infundibular septum, a process that completes the spiral division of the truncus arteriosus and establishes left ventricular origin of the aorta and right ventricular origin of the pulmonary trunk. The aortic and pulmonary valves and their sinuses develop from truncal tissue at the line of fusion of the truncal and infundibular septa. In persistent truncus arteriosus, the truncal septum fails to develop. The infundibular septum is deficient or absent, which is responsible for a nonrestrictive ventricular septal defect roofed by the truncal valve. Vestigial development of the distal truncal septum is responsible for a short main pulmonary artery that arises from the truncus. When the truncal septum is absent altogether with no vestigial remnant, the main pulmonary artery is also absent, so the right and left pulmonary arteries arise directly from the truncus by separate ostia.
Physiologic consequences of truncus arteriosus depend on the size of the pulmonary arteries and on the pulmonary vascular resistance. Right ventricular pressure is identical with systemic because both ventricles communicate directly with the biventricular truncus via the nonrestrictive ventricular septal defect. When a main pulmonary artery arises directly from the truncus, blood flow from the left and right ventricles tends to cross, so oxygen content is higher in the aorta than in the pulmonary artery. Systemic arterial oxygen saturation is high when pulmonary resistance is low and pulmonary blood flow is increased, an advantage purchased at the price of volume overload of the left ventricle and congestive heart failure. Truncal valve regurgitation or truncal valve stenosis adds to the hemodynamic burden of the volume-overloaded left ventricle4,30 and to the hemodynamic derangements that are imposed on the right ventricle because of the biventricular truncus. As pulmonary vascular resistance rises, pulmonary blood flow falls. Volume overload of the left ventricle is curtailed at the price of increased cyanosis. Occasionally, pulmonary blood flow is adequately regulated by mild to moderate hypoplasia of both pulmonary arteries. In patients with truncus arteriosus and an absent right or left pulmonary artery, early vascular disease develops in the contralateral pulmonary artery (see Figure 28-16A).22
History
Truncus arteriosus occurs with equal frequency in males and females.17 Isolated examples have been reported in siblings17,45–47 and in twins,48–50 and a relatively high incidence rate of congenital cardiac malformations is found in siblings of children with truncus arteriosus. Familial recurrence of nonsyndromic truncus with interrupted aortic arch has been described.51 Truncus arteriosis has been reported with chromosome 22q11 deletion,15,52 trisomy 18,53 and trisomy 21.
Truncus arteriosus comes to attention in the first few weeks of life because of tachypnea, diaphoresis, poor feeding, and failure to thrive. As pulmonary resistance falls, pulmonary blood flow increases and neonatal cyanosis diminishes or may virtually vanish. Truncal valve regurgitation is responsible for biventricular failure because regurgitant flow is received by both ventricles. A systolic murmur awaits the fall in neonatal pulmonary vascular resistance analogous to the time course with ventricular septal defect (see Chapter 17).
Infants with truncus arteriosus seldom reach their first birthday.5,17,54 Most die of congestive heart failure in the first few months of life. In van Praagh and van Praagh’s7 necropsy series, mean age at death was 5 weeks. A rise in pulmonary vascular resistance occasionally regulates pulmonary blood flow and relieves the volume-overloaded left ventricle, so symptoms of congestive heart failure improve while cyanosis deepens. A small but not insignificant number of patients reach their third, fourth, or fifth decade (see Figures 28-15 through 28-18),5,54–57 with an occasional survival into the sixth58,59 or seventh decade.60 A patient with truncus arteriosus “type 4” survived to age 54 years.61 Patients with truncus arteriosus and Eisenmenger’s syndrome are subject to the multisystem systemic disorders described in Chapter 17. Rarely, death is from sudden intramural dissection and rupture of the common trunk.62 Morbidity and mortality are also influenced by a host of noncardiac abnormalities that coexist with truncus arteriosus, as described subsequently.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree