Tricuspid Valve Repair and Replacement
Patrick M. McCarthy
Jennifer Higgins
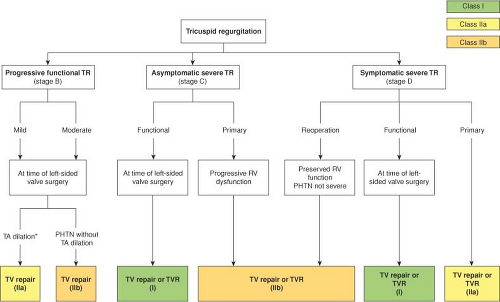
The indications to perform tricuspid valve surgery have been vague in the past but have become better defined as there are more publications about tricuspid surgery indications and outcomes, and European and U.S. guidelines are more clear. Surgical decision making is straightforward if one follows the guidelines (Fig. 22.1). For patients undergoing left-sided valve surgery (most commonly, mitral valve) with severe tricuspid regurgitation it should be repaired regardless of symptoms (Class I). For patients with mild or moderate tricuspid regurgitation undergoing left-sided valve surgery, the indications have become more liberal over time, based on recent literature. For those with tricuspid annular dilatation (>40 mm by TTE or >21 mm/m2) TV repair is a Class IIa recommendation. For patients with pulmonary hypertension without annular dilation TV repair is a Class IIb recommendation. For symptomatic severe TR in the setting of a reoperation (usually from unrepaired moderate TR at the time of MV surgery) it is a IIb recommendation to perform TV repair or replacement. The surgeon must be careful and use good judgment in these patients because many patients are not referred until hepatic and/or renal dysfunction has begun, and patients also may have developed clinically important and irreversible RV dysfunction. Finally, for patients with severe primary TR (e.g., endocarditis, ruptured chords from trauma) if they are symptomatic that is a Class I indication, and asymptomatic but with progressive RV dysfunction is a Class IIb indication, for TV repair or replacement. Although this is better than before, the 2014 guidelines section on Tricuspid Disease is only 6 pages of the 132 page document. Of course, these are only guidelines, and occasionally a surgeon may choose to be more conservative depending upon the overall comorbidities and complexity of the operation.
Surgical Anatomy of the Tricuspid Valve
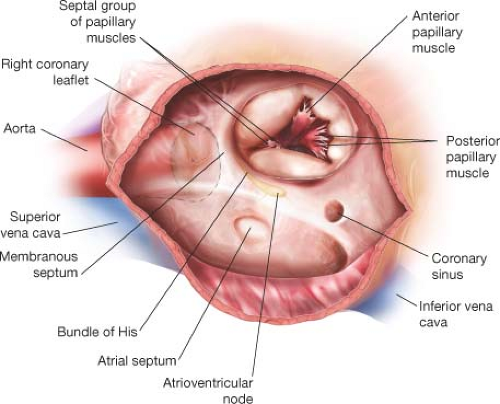
The tricuspid valve is composed of the annulus, three leaflets (anterior, posterior, and septal), and the subvalvular apparatus (anterior, posterior, and septal) papillary muscles with chordae tendinae (Fig. 22.2). The anterior papillary muscle is usually dominant, with two or three separate attachments to the anterior ventricle. It arises near the apex,
with single or multiple heads. The posterior papillary muscle can be single or double, and is implanted on the posterior RV wall. The septal group is comprised of several small papillary muscles implanted on the septum and conus. Primary, secondary, and tertiary chordae connect the papillary muscles to the tricuspid valve leaflets, usually with 3 mm or less between adjacent chordae. Chordae from the anterior papillary
muscle extend to the anterior commissure and the adjacent anterior and posterior leaflets. Chordae from the posterior papillary muscle extend to the posterior leaflet and the posteroseptal commissure. Chordae arising from the septal group attach to the septal leaflet and adjacent commissures.
with single or multiple heads. The posterior papillary muscle can be single or double, and is implanted on the posterior RV wall. The septal group is comprised of several small papillary muscles implanted on the septum and conus. Primary, secondary, and tertiary chordae connect the papillary muscles to the tricuspid valve leaflets, usually with 3 mm or less between adjacent chordae. Chordae from the anterior papillary
muscle extend to the anterior commissure and the adjacent anterior and posterior leaflets. Chordae from the posterior papillary muscle extend to the posterior leaflet and the posteroseptal commissure. Chordae arising from the septal group attach to the septal leaflet and adjacent commissures.
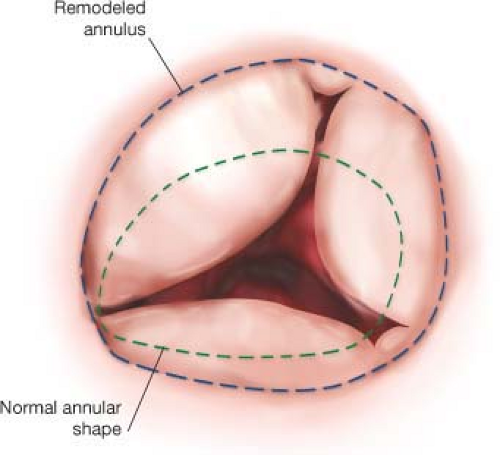
The tricuspid valve has a unique three-dimensional structure, better defined with the advent of three-dimensional echocardiography and CT scan techniques (Fig. 22.3). The highest point of the tricuspid annulus is along the base of the anterior leaflet at the RV outflow tract and the lowest point is along the septal leaflet adjacent to the bundle of His (anteroseptal commissure) and atrioventricular node. The tricuspid circumference has been reported to be 9.3 +/− 0.9 cm and this decreases by 13 +/− 1% during systole. The orifice area during diastole is 7.1 +/− 1.3 cm2 and during systole is 5 +/− 1 cm2. During systole the annulus achieves an ovoid shape with a greater transverse diameter. In the clinical setting of functional TR with pulmonary hypertension and RV dilatation the annulus assumes a circular shape with most of the annular dilatation found at the base of the anterior and posterior leaflets.
Within the right atrium, the smooth posterior groove extends from the SVC to the IVC. This smooth wall of the atrium is separated from the trabeculated dome by a ridge of muscle, called the crista terminalis. Superiorly, the crista terminalis arches anterior to the orifice of the SVC, extends to the area of the interatrial groove, and merges with the interatrial bundle, known as the Bachmann bundle. Changes in the musculature of the Bachmann bundle could block or prolong interatrial conduction, resulting in abnormal atrial excitability, atrial dysfunction, atrial fibrillation, and other arrhythmias. The most important landmark that the surgeon has to keep in mind is the conduction system (Fig. 22.2). The conduction system must be avoided during suture placement for repair or replacement. The coronary sinus, the tendon of Todaro, and the septal leaflet comprise the triangle of Koch, and the conduction system is at the apex of this triangle. When placing sutures, the surgeon should avoid placing any sutures near the apex of the triangle of Koch. We place sutures above the anterior septal commissure, and typically the sutures end along the medial one-third of the septal leaflet at approximately the level of the coronary sinus. The surgeon also should be aware of the close proximity of the aortic sinuses to the tricuspid valve. When performing tricuspid valve surgery with the cross-clamp off, the bulge of the aortic sinuses is evident and makes the surgeon more conscious of this relationship and less likely to place a full-thickness suture that could cause bleeding from the aortic wall. The right coronary artery occasionally runs close to the anteroposterior commissure and leaflets and could be injured.
Careful inspection of the tricuspid annulus, leaflets, and subvalvular apparatus is performed to confirm the preoperative echocardiographic findings. “Functional” tricuspid regurgitation (Type I annular dilatation) is the most common cause of TR, and is found in patients with left-sided valve disease, pulmonary hypertension, long-standing persistent atrial fibrillation, or cardiomyopathy (Fig. 22.4). The right ventricle may be dilated, leading to tethering of the tricuspid leaflets and annular dilation. Severe leaflet tethering has been reported to be a significant risk factor for tricuspid repair failure, and when extreme leads us to consider tricuspid valve replacement (TVR) or patch enlargement of the anterior leaflet. Most pathologic annular dilatation occurs along the anterior and posterior leaflet. Some patients have marked dilation of the commissure
between the anterior and posterior leaflets leading to what appears to be a large cleft, although it is simply the commissure distorted by the pathology. Some patients may have other tricuspid valve lesions, caused by prolapse, endocarditis with vegetations or perforations, rheumatic disease with subvalvular fusion leaflet thickening and retraction, and carcinoid valve disease with extensive fibrosis, thickening, and shortening of the entire valve and subvalvular complex.
between the anterior and posterior leaflets leading to what appears to be a large cleft, although it is simply the commissure distorted by the pathology. Some patients may have other tricuspid valve lesions, caused by prolapse, endocarditis with vegetations or perforations, rheumatic disease with subvalvular fusion leaflet thickening and retraction, and carcinoid valve disease with extensive fibrosis, thickening, and shortening of the entire valve and subvalvular complex.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree