TRICUSPID ATRESIA
Introduction
Continuing the same theme as in the preceding 2 chapters, the author will discuss tricuspid atresia (TA) in this chapter. For the readers interested in more detailed discussion of this lesion, they are referred to my books1,2 and comprehensive reviews published elsewhere.3–5
TA is a cyanotic, congenital heart defect (CHD) and is defined as congenital absence or agenesis of the morphologic tricuspid valve (TV).6,7 It is the third most common cyanotic CHD and is the most common cause of cyanosis with left ventricular (LV) hypertrophy. Although there is a controversy with regard to terminology, whether it should described as TA, univentricular heart, or univentricular connection, the author’s view is that the term “tricuspid atresia” is the proper and rational term to characterize this well-described pathological and clinical entity; the reasons for this conclusion are detailed in our prior publications.7–9
The exact prevalence of TA is unknown. Extensive evaluation of the published literature revealed a prevalence of 2.9% in autopsy cases and a prevalence of 1.4% in clinical cases of CHD.10 The prevalence of TA in neonates with CHD is also very similar at 1.5%.11 Given the prevalence of CHD of 0.8% of live births, it may be projected that TA occurs approximately 1 in 10,000 live births.10 While there is no gender predominance for all TA cases, male preponderance seems to be present in TA patients with transposition of the great arteries (TGA); male to female ratio was approximately 2:1.10,12
Classification
The classifications of TA have utilized valve morphology,13 pulmonary vascular markings on chest roentgenogram,14 and associated cardiac defects6,15–18 in an attempt to characterize the defect.
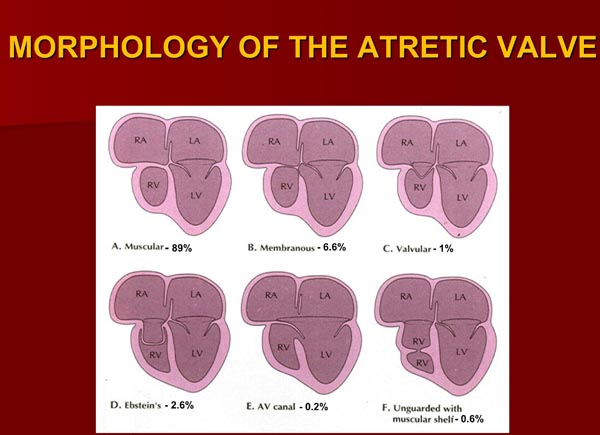
Based on the morphologic appearance of the atretic TV, it has been classified into muscular, membranous, valvular, Ebstein’s, unguarded with muscular shelf, and atrioventricular (AV) canal types.13,19–23 The muscular type, constitutes 89% of cases18,20,23 and is the most common type; other types occur with much lower frequency (Figure 30.1).
Figure 30.1. Diagrammatic portrayal of anatomic types of TA based on the morphology of the atretic TV. A: muscular type; B: membranous type; C: valvular type; D: Ebstein’s type; E: AV canal type; and F: unguarded valve with muscular shelf. The prevalence of each is shown under each diagram. For the sake of simplicity, great vessels are not shown. Also note that no VSDs are shown. LA, left atrium; LV, left ventricle; RA, right atrium; RV right ventricle. Modified from Neonatology Today 2012;7(5):1–12.
Classification based exclusively on the x-ray appearance of pulmonary vascular markings12,14 divides them into Group A: decreased pulmonary vascular markings, Group B: increased pulmonary vascular markings, and Group C: transition from increased to decreased pulmonary vascular markings in serial chest films.
The above 2 classifications do have some clinical value; however, classification based on associated cardiac defects seems to be more useful clinically.6,18 Initially, a classification depending on interrelationship of the great arteries was proposed by Kühne in 1906.15 This classification was later advanced by Edwards and Burchell16 as well as by Keith et al.17,21,22 However, there were some inconsistencies in subgrouping and there was a need for inclusion of every variation in great artery anatomy. Therefore, we proposed a new (at that time) and unified classification,6 which is shown in Table 30.1.
Table 30.1. Classification of Tricuspid Atresia
| Type I | Normally related great arteries | |
| Type II | d-TGA | |
| Type III | Malpositions of the great arteries other than d-TGA | |
| Subtype 1. L-TGA Subtype 2. Double-outlet right ventricle (DORV) Subtype 3. Double-outlet left ventricle (DOLV) Subtype 4. D-malposition of the great arteries (anatomically corrected malposition) Subtype 5. L-malposition of the great arteries (anatomically corrected malposition) | ||
| Type IV. Persistent TA | ||
| Each Type and Subtype are further divided | ||
| Subgroup a. Pulmonary atresia Subgroup b. Pulmonary stenosis or hypoplasia Subgroup c. Normal PAs (no pulmonary stenosis) | ||
Initially, the TA was divided into 4 major types depending on the relationship of the great arteries, namely: Type I, normally related great arteries; Type II, d-TGA; Type III, malpositions of the great arteries other than d-TGA; and Type IV, truncus arteriosus. The Type III is again subclassified into several subtypes (see Table 30.1). Each of the types and subtypes are again divided into subgroups on the basis of pulmonary arteries: Subgroups a, pulmonary atresia; b, pulmonary stenosis or hypoplasia; and c, normal pulmonary arteries (no pulmonary stenosis).6,18,20,23 After classifying into types and subgroups, the status of the ventricular septum, that is, intact, small, or large ventricular septal defect (VSD), or multiple VSDs as well as other associated defects are enumerated. If the clinician desires to use the terminology of CHD devised and reemphasized by Van Praagh,24 the remaining segmental subsets, namely visceroatrial situs and ventricular loop, may be included; each case could be depicted by notations {S,D,S}, {S,D,D}, {S,D,L}.24,25
Pathologic Anatomy
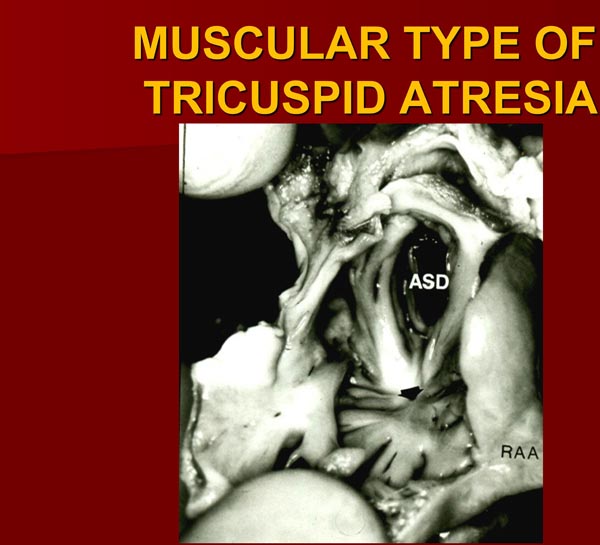
The right atrium (RA) is enlarged and the TV is atretic. In the most common muscular variety, atretic TV is seen as a dimple or a localized fibrous thickening in the floor of the RA (Figure 30.2) at the anticipated site of the TV.17 No valvar material can be recognized both on gross and microscopic examination.17 Other anatomic types (membranous,26,27 valvular,17,27–29 Ebstein’s,12,13,26,30 AV canal,19,31 and unguarded with muscular shelf32) have characteristic appearances and are diagrammatically portrayed in Figure 30.1.
Figure 30.2. Heart specimen of a patient with muscular type of TA; the RA is opened by cutting through the RA appendage (RAA). Note dimple (arrow) in the floor of the RA with muscle fibers radiating around it. An ASD is also shown. The impression that one gets from the literature is that this dimple is present in most cases of TA. Careful inspection of the heart specimens by several investigators suggest that this dimple is seen in only 29% to 83% of muscular-type of TA cases. Reproduced with permission from Neonatology Today 2012;7(5):1–12.
An interatrial defect is necessary for survival and is typically a stretched patent foramen ovale (PFO). However, occasionally an ostium secundum atrial septal defect (ASD) or an ostium primum ASD may be present facilitating right-to-left shunt. The left atrium (LA) is enlarged, especially when the pulmonary blood flow (PBF) is increased. The mitral valve (MV) is usually bicuspid and morphologically a MV. However, its orifice is large and occasionally incompetent. The LV is evidently a morphological LV, but it is enlarged and hypertrophied. The right ventricle (RV) is hypoplastic and is not sufficiently large in size to support pulmonary circulation.
The ventricular septum is intact in a rare patient, but usually a VSD is present; this may be large, small, or multiple VSDs may be seen. The VSD may be conoventricular or perimembranous, located inferior to the septal band; conal septal malalignment type, located in between the anterosuperior and posteroinferior limbs of septal band; muscular, located inferiorly in the muscular septum; or rarely of AV canal type.25 In our personal experience, the VSDs are most commonly muscular.33–36 These VSDs are usually restrictive and cause subpulmonary obstruction in the Type I patients and subaortic stenosis in the Type II patients.33–41
The great vessel relationship is variable, as discussed in the classification section. The ascending aorta (AAo) is either normal or slightly larger than normal. Right ventricular outflow tract (RVOT) is atretic, narrowed, or normal, depending on the subgroup (a, b, and c). In patients with normally related great arteries (Type I), the RV outflow obstruction is frequently at the VSD level although subvalvar or valvar (very rare) pulmonary stenosis or narrow tract of the hypoplastic RV may occasionally be responsible for such obstruction. In cases with TGA (Type II), the obstruction is usually subvalvar. In cases with pulmonary atresia, either a patent ductus arteriosus (PDA) or aortopulmonary collateral vessels are present.
Other abnormalities may be present in nearly 30% of TA cases42,43; significant among these are coarctation of the aorta (CoA), particularly in Type II (transposition) cases and persistent left superior vena cava (PLSVC), which have therapeutic implications.
Pathophysiology
During Prenatal Period
In the fetus, TA is not detrimental to normal fetal development. In the TA fetus with intact ventricular septum and/or pulmonary atresia (Types Ia and IIa), the PBF is wholly supplied by the ductus arteriosus (DA). Because the ductus is transporting only the PBF, accounting for 8% to 10% of combined ventricular output when compared with 66% in the normal fetus,44,45 the DA is expected to be smaller than that seen in the normal fetus. Also, because of reversal of direction of ductal flow, there is acute angulation of the ductus at the aortic origin.
In the fetus with normally related great arteries with VSD (Types Ib and Ic), the quantity of blood flow from the LV through the VSD into the RV and the pulmonary artery (PA) and from there into the DA, versus the amount of blood flow retrograde from the Ao to the DA, varies with size of the VSD. The smaller the VSD, the lesser is the magnitude of anterograde ductal flow.
In the fetus with Type I anatomy with a small or no VSD, the majority of the LV blood is ejected into the Ao, which is then transported to the entire body, including the placenta. Consequently, a larger proportion of ventricular output (than in a normal fetus) is transported across the aortic isthmus; this is thought to be the reason for the rarity of aortic coarctation in these subgroups of TA babies. In contrast, in a Type II (transposition) fetus without pulmonary stenosis, a larger proportion of blood traverses the PA and DA to reach the descending aorta (DAo), and therefore the aortic isthmus flow is low. This may account for higher frequency of CoA with these types of TA.
During Postnatal Period
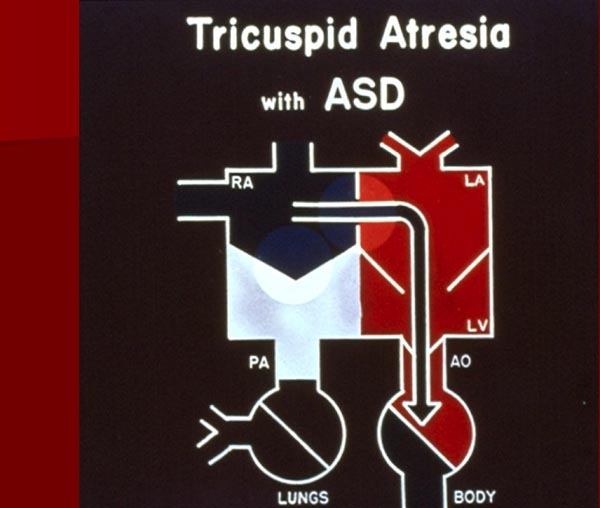
Because of the atretic TV, the entire systemic venous return is transmitted across the atrial septum either via PFO or ASD. This obligatory right-to-left shunt mixes with pulmonary venous return in the LA (Figure 30.3). The mixed pulmonary and systemic venous (including coronary) blood enters the LV.
Figure 30.3. Box diagram of TA. The systemic venous return cannot exit into the RV, and its egress has to be into the (LA) via a PFO or an ASD. Then, the blood traverses into the LV and Ao and body. See Figure 30.4 for further description of blood flow in TA. RA, right atrium, PA, pulmonary artery. Reproduced with permission from Neonatology Today 2012;7(5):1–12.
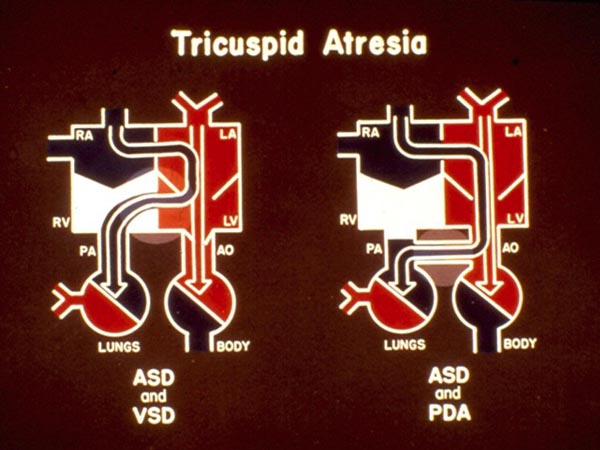
In babies with normally related great arteries and a VSD (Types Ib and Ic), left-to-right shunt occurs across the ventricular septum with resultant perfusion of the lungs (Figure 30.4, left panel). If there is no VSD, the lungs are perfused either via a PDA (Figure 30.4, right panel) or through aortopulmonary collateral vessels (not shown). The existence of either a VSD or other means of blood supply to the lungs is critical for the baby’s survival. The blood flow into the Ao is derived directly from the LV, a mixture of pulmonary and systemic venous returns.
Figure 30.4. Box diagrams of TA: left panel with VSD and the right panel with PDA. As seen in Figure 30.3, the RA blood exits into the LA, mixes with pulmonary venous return then into the LV. In the presence of a VSD (left panel), shunting from the LV to RV takes place, thus perfusing the PA and the lungs. In the absence of VSD but with PDA (right panel) left-to-right shunting occurs at the ductal level perfusing the PA and the lungs. The presence of VSD, PDA, or aortopulmonary collateral vessels is critical for patient survival. Reproduced with permission from Neonatology Today 2012;7(5):1–12.
In babies with d-TGA (Type II), the PBF directly comes from the LV. The systemic blood flow is through the VSD and the RV.
In Type III, Subtype 1 with l-TGA, the atretic morphologic TV is on the left side. Consequently, it is mitral (pulmonary venous atrial) valve obstruction. In other Type III and Type IV46 babies, the systemic and pulmonary blood flows are largely determined by the size of the VSD and other associated defects.
The above-described pathophysiology results in arterial desaturation and LV volume overloading. The arterial desaturation is related to complete mixture of the systemic, coronary, and pulmonary venous blood in the LA and the LV. The LV volume overloading occurs because the entire systemic, coronary, and pulmonary venous blood returns have to be pumped by the LV; this is further increased if the PBF is increased, either because of mild or no obstruction to pulmonary outflow flow tract or because of large, surgically created shunts. Both these tend to produce heart failure.
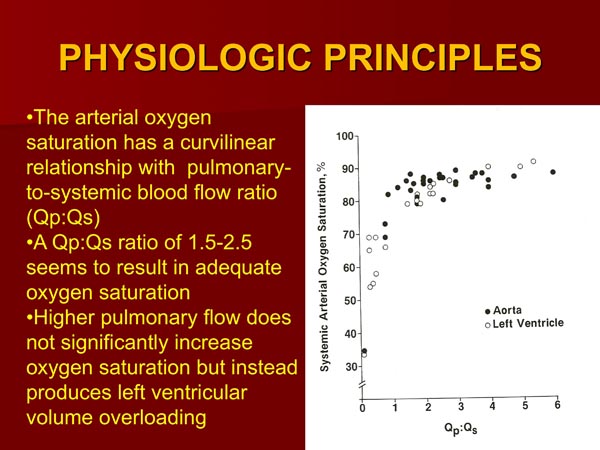
The oxygen saturation is proportional to the amount of the PBF.44,47 The quantity of PBF is inversely proportional to the degree of obstruction of the pulmonary outflow tract and directly proportional to the size of PDA when such is present. If a systemic-to-pulmonary artery shunt had been performed by surgery, the PBF is proportional to the size of the shunt. The pulmonary-to-systemic blood flow ratio (Qp:Qs) which represents the PBF has a curvilinear relationship with the arterial oxygen saturation (Figure 30.5). A Qp:Qs of 1.5:2.5 results in an adequate systemic arterial oxygen saturation.47 Additional increase in Qp:Qs not only does not produce better oxygen saturation, but also subjects the LV to larger volume overloading and, for that reason is not advisable.47
Figure 30.5. The systemic arterial saturations (LV or Ao) in patients with TA are plotted against the respective pulmonary to systemic blood flow ratios (Qp:Qs). Both Type I and Type II anatomy are included. Note curvilinear relationship between 2 parameters. At low Qp:Qs levels, a slight increase in Qp:Qs produces large increase in systemic O2 saturation, whereas at higher Qp:Qs, further increase does not produce significant increase in O2 saturation. Ideal Qp:Qs appears to be between 1.5 to 2.5, giving O2 saturations in low 80s. Aortic saturations are marked as solid circles and LV saturations as open circles. Reproduced with permission from Neonatology Today 2012;7(5):1–12.
Changing Hemodynamics
The pathophysiology also evolves with time; these changing hemodynamics are discussed below.
PDA Spontaneous closure of the DA may occur in the early neonatal period and results in severe hypoxemia. Prostaglandin E1 (PGE1) infusion may help open the ductus.
PFO Since the entire systemic venous return must traverse through the PFO, any restriction of this structure causes systemic venous congestion and diminished cardiac output (CO). However, very few patients with TA have clinically significant obstruction,12 especially in the early neonatal period. A tall “a” wave in the RA and a mean interatrial pressure difference more than 5 mmHg is indicative of an interatrial obstruction. Sometimes, balloon atrial septostomy may be required to relieve this obstruction.
VSD The VSD may close spontaneously,33–41 resulting in decreased PBF and hypoxemia in Type I (normally related great arteries) TA babies and subaortic obstruction in Type II (transposed great arteries) infants. Bypassing the obstructed VSD surgically may be required. However, such VSD closures happen over a period of months and years and are not relevant to our review of TA in neonates. For further discussion of this subject, the reader is referred to other publications.35,36,40,43
Clinical Features
The major determinant of clinical features of TA is the quantity of PBF. A baby with markedly reduced PBF is likely to present in the first few days of life with severe cyanosis and hypoxemia. On the contrary, a baby with markedly increased PBF usually presents with signs of heart failure in the first few weeks of life and does not have significant cyanosis. While there is some overlap, infants with decreased pulmonary flow typically belong to Type I with normally related great arteries and those with increased PBF generally belong to Type II with TGA and rarely Type Ic.
Roughly one-half of the patients with TA manifest symptoms on the first day of life, and 80% of the neonates would become symptomatic by the end of the first month of life.12,48 As mentioned in the preceding paragraph, 2 forms of presentation are observed: (1) decreased PBF and (2) increased PBF.
Decreased PBF
Babies with decreased PBF present with symptoms early; the more severe the pulmonary oligemia, the earlier the clinical presentation. The babies with hypoxemia may have hyperpnea and acidosis. The majority of these infants belong to Type Ib. Babies with pulmonary atresia (Subgroup a) independent of the type will also present as the ductus begins to constrict. Hypercyanotic spells may occur later in infancy, but are not common in the neonate.
Physical examination shows central cyanosis, tachypnea or hyperpnea, normal pulses, normal ventricular impulses, no precordial thrills, and a single second heart sound. A holosystolic murmur, typical for a VSD is heard at the left lower or left mid-sternal border. No diastolic murmurs are usually heard. Babies with pulmonary atresia do not usually have murmurs; however, on occasion a continuous murmur of PDA may be auscultated. No clinical signs of congestive heart failure (CHF) are present. If there is significant interatrial obstruction, prominent “a” waves in the jugular venous pulse may be seen and presystolic hepatic pulsations may be felt.
Increased Pulmonary Flow
Babies with increased PBF generally present with signs of CHF within the first few weeks of life; the presentation is much later than that seen in babies with pulmonary oligemia. However, a rare infant may present within the first week of life,11 presumably related to the rapid fall in pulmonary vascular resistance. These infants are only mildly cyanotic, but have symptoms of dyspnea, fatigue, difficulty to feed, and increased perspiration. Repeated respiratory tract infection and failure to gain weight are other types of presentation. Most of these babies belong to Type IIc, while a small percentage of patients may belong to Type Ic. There is a higher prevalence of aortic coarctation in Type II patients, as mentioned above, and when present, early cardiac failure occurs with coarctation.
Physical examination reveals tachypnea, tachycardia, intercostal or subcostal retractions, minimal cyanosis, and hepatomegaly. The cardiac impulses are increased and hyperdynamic. On auscultation, a single (occasionally split) second heart sound heard best at left upper sternal border, a holosystolic murmur of VSD typically heard at the left lower sternal border, and a mid-diastolic murmur heard best at the apex are appreciated. Definitive signs of congestive cardiac failure are frequently present. Decreased femoral pulses are seen when there is associated aortic coarctation but without a significant-sized PDA. When there is an interatrial obstruction, prominent “a” waves in jugular veins and/or presystolic hepatic pulsations may be present.
Noninvasive Evaluation
Chest x-ray
By and large, radiographic appearance is dependent on the total PBF (Figure 30.6). The heart size is either normal or minimally enlarged in babies with decreased pulmonary flow, while the heart size is moderate to severely enlarged in infants with increased PBF. Several cardiac configuration patterns such as “characteristic” TA appearance,49 coeur en sabot configuration,50 “egg-shaped”,22 “bell-shaped”51 and square14 heart have been mentioned, but in the author’s personal experience as well as that of others,22 there is no dependable pattern that would be diagnostic of TA. The RA outline may be prominent. Concavity in the region of PA segment is seen in babies with decreased PBF and small PA.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree