Chapter 25 Tricuspid Atresia
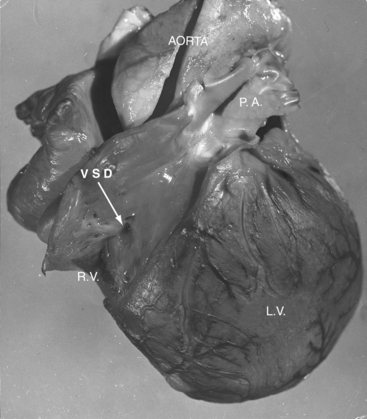
Tricuspid atresia was described in 1817,1 but almost a century elapsed before the great arterial relationships were defined.2 Because of the morphologic heterogeneity of the malformation, “manifold anatomic combinations can result in this haemodynamic arrangement.”3,4 The incidence rate has been estimated at 0.06 per 1000 live births, with a prevalence rate of 1% to 3% of congenital heart disease.5,6 An unguarded tricuspid orifice is different again.7 This chapter is concerned with hearts in situs solitus without ventricular inversion in which a physiologic or anatomic connection does not exist between the morphologic right atrium and the morphologic right ventricle. In 95% of patients, absence of the right atrioventricular connection is the result of fibro-fatty tissue that is interposed between the muscular floor of the right atrium and the parietal wall of the ventricular mass.8 In the remaining 5%, atresia is produced by an imperforate tricuspid valvular membrane.4,8 Systemic venous return cannot directly reach the ventricular portion of the heart but instead crosses the atrial septum from a morphologic right atrium into a morphologic left atrium, where it mixes with pulmonary venous return before traversing a solitary atrioventricular valve into a morphologic left ventricle, which is the only pumping chamber for the pulmonary and systemic circulations (Figure 25-1).3 This arrangement has been referred to as a “functionally” univenricular heart.4 Atresia of the tricuspid valve with Ebstein’s anomaly and atresia of the right atrioventricular valve with single ventricle are dealt with in Chapters 13 and 26.
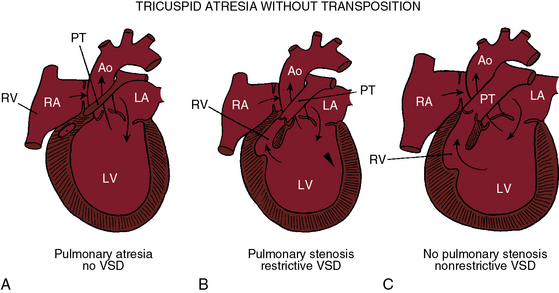
Tricuspid atresia, as just defined, has certain anatomic features that consistently recur and certain features that are variable.6,9,10 Consistent features include: (1), physiologic and anatomic absence of a connection between the morphologic right atrium and the morphologic right ventricle; (2), hypoplasia of the morphologic right ventricle; (3), an interatrial communication; and (4), a morphologic left ventricle equipped with a morphologic mitral valve. The variable features provide the rationale for a clinical classification based on three gross morphologic features4,6,9–11: (1), transposed or nontransposed great arteries; (2), the presence or absence of pulmonary stenosis (Figures 25-2 and 25-3); and (3), the size of the coexisting ventricular septal defect. An embryologic classification is based on two microscopic features: (1), rudiments of an atretic tricuspid apparatus that create a dimple on the floor of the right atrium that can be localized with transillumination by a light source placed within the hypoplastic right ventricle10,12,13; and (2), a fibrous atrioventricular remnant that forms a microscopic tract from the right atrium to a tiny inlet component of the subjacent right ventricle.14 Failure of expansion of this exceedingly small inlet component during early embryogenesis is believed to be the pathogenetic mechanism responsible for most cases of tricuspid atresia.14 Congenital tricuspid stenosis is a less severe form of the malformation in which a well-formed tricuspid valve joins the small inlet portion of the right ventricle (Figure 25-4).12
In approximately three fourths of cases, an interatrial communication exists in the form of a restrictive patent foramen ovale.15 The valve of the foramen ovale occasionally protrudes aneurysmally as the obstructed right atrium vainly seeks an exit.16,17 The aneurysmal protrusion can obstruct left atrial flow.17 A much less common form of interatrial communication is an atrial septal defect that is almost always ostium secundum.9,15
The great arteries are nontransposed in approximately 90% of cases.11,15 Pulmonary blood flow then depends on the condition of the ventricular septum (see Figure 25-2). The arrangement at birth is usually a restrictive ventricular septal defect (see Figure 25-2B) that constitutes a zone of subpulmonary stenosis, which is physiologically advantageous when blood flow to the lungs is adequate but not excessive. The advantage is lost in about 40% of cases because the defect decreases in size or closes altogether—acquired pulmonary atresia (see Figures 25-2A, and 25-6).15,18–20 The time course of spontaneous closure is similar to that of isolated perimembranous ventricular septal defects (see Chapter 17), with most that are destined to close doing so in the first year of life.15,18–20 Rarely, the ventricular septum is congenitally intact and the pulmonary valve is atretic, an arrangement that completely denies the left ventricle access to the pulmonary circulation (see Figure 25-2A). Also rarely, obstruction is exclusively at valve level because a bicuspid pulmonary valve is stenotic.21 A nonrestrictive ventricular septal defect (Figures 25-2C, and 25-5) permits unobstructed flow from left ventricle to main pulmonary artery. The right ventricle is well-developed, and the pulmonary valve is normally formed. Pulmonary blood flow is regulated by pulmonary vascular resistance.6
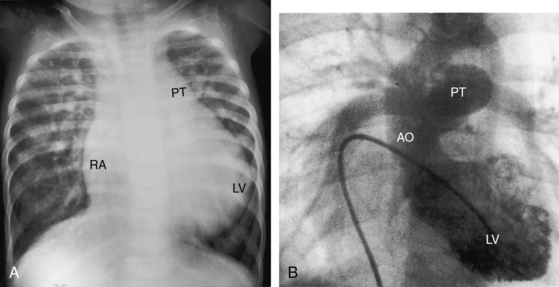
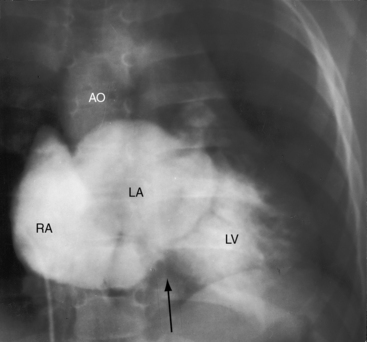
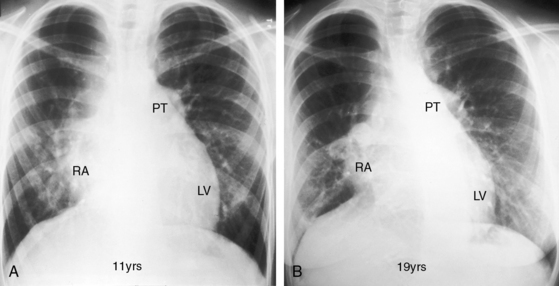
Figure 25-5 A, X-ray from a 6-year-old girl with tricuspid atresia, normally related great arteries, a nonrestrictive ventricular septal defect, low pulmonary vascular resistance, and a large ostium secundum atrial septal defect (see Figure 25-2C). Pulmonary blood flow is markedly increased, the pulmonary trunk (PT) is prominent, the right atrium (RA) is enlarged, and a dilated left ventricle (LV) occupies the apex. B, Left ventriculogram from a 4-year-old girl with tricuspid atresia, normally related great arteries, a nonrestrictive ventricular septal defect, and low pulmonary vascular resistance. The pulmonary trunk (PT) and its branches are dilated, and the left ventricle is enlarged. Compare with Figure 25-2C.
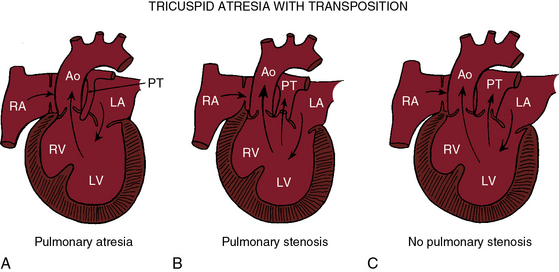
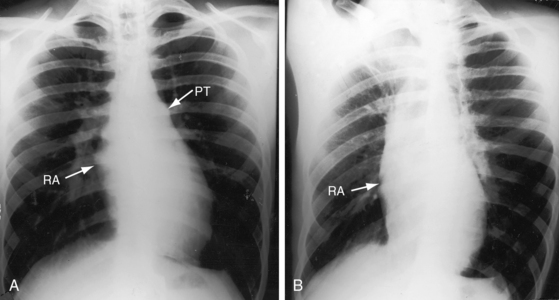
Tricuspid atresia with complete transposition of the great arteries typically occurs with a nonrestrictive ventricular septal defect without pulmonary stenosis (see Figure 25-3).11,15 Left ventricular blood has unobstructed access to the transposed aorta through a well-developed right ventricle. Pulmonary blood flow is regulated by pulmonary vascular resistance because the transposed pulmonary trunk originates from the left ventricle (see Figure 25-3). Pulmonary vascular disease usually develops in the first year of life (Figures 25-7 and 25-8).15 A decrease in size or spontaneous closure of the ventricular septal defect constitutes a zone of subaortic stenosis because the transposed aorta arises from the right ventricle.22 Pulmonary stenosis is infrequent, and pulmonary atresia is rare (see Figure 25-3).23
Distribution of the coronary arteries in tricuspid atresia is analogous to, if not identical with, that of univentricular hearts with a single morphologic left ventricle and an outlet chamber (see Chapters 26 and 32).24 The rudimentary right ventricle of tricuspid atresia and the right ventricular remnant of single ventricle are both delimited by coronary arteries.24
Additional anatomic variables associated with tricuspid atresia involve the mitral valve,25 the ductus arteriosus, the ascending aorta, the aortic isthmus, the atrial appendages, and the pulmonary valve.26 Abnormalities of the mitral valve are represented by myxomatous, redundant, or prolapsing leaflets; a cleft anterior leaflet; and direct attachment of leaflets to papillary muscles.25 When the great arteries are nontransposed and the ventricular septum is congenitally intact, which is physiologic pulmonary atresia, the fetal ductus arteriosus functions as a small malformed aortic tributary.27 The ascending aorta and isthmus are large because the aorta receives the entire cardiac output. When the great arteries are transposed and the ventricular septal defect is restrictive, left ventricular blood is diverted into the pulmonary trunk, so the ductus arteriosus enlarges while the ascending aorta and isthmus are underfilled and hypoplastic.15,28 Very rarely, the pulmonary valve is absent.26 Juxtaposition of the atrial appendages, a condition in which both appendages lie on one side of the great arteries,15,29–31 almost always means that the great arteries are transposed (see Chapter 27). Juxtaposition is present in about 50% of patients with tricuspid atresia and complete transposition.
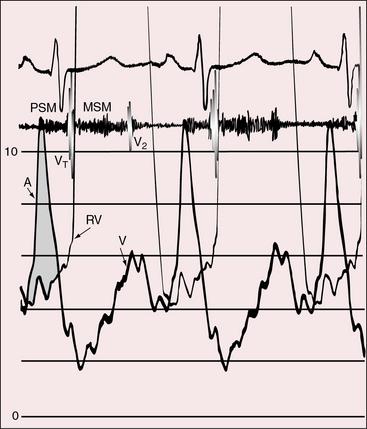
The physiologic consequences of tricuspid atresia begin with the obligatory right-to-left shunt at the atrial level. The left atrium receives the normal pulmonary venous return together with the systemic venous return across the interatrial communication.32 The left atrial mixture flows across a morphologic mitral valve into a morphologic left ventricle, which is the sole pumping chamber for the systemic and pulmonary circulations. When the great arteries are not transposed, pulmonary blood flow is reduced because a restrictive ventricular septal defect constitutes a zone of subpulmonary stenosis (see Figures 25-2 and 25-6). This arrangement accounts for about 90% of cases. Left ventricular volume overload is curtailed at the price of increased cyanosis. When the ventricular septal defect is nonrestrictive and pulmonary vascular resistance is low, pulmonary blood flow and left ventricular volume overload are excessive, and cyanosis is mild (see Figure 25-5). When the great arteries are transposed, the ventricular septal defect is usually nonrestrictive and pulmonary stenosis is usually absent (see Figure 25-3). Low pulmonary vascular resistance results in increased pulmonary blood flow, mild cyanosis, and left ventricular volume overload.33,34 The degree of pulmonary vascular resistance that achieves adequate but not excessive pulmonary blood flow is a delicate balance that is seldom realized (see Figures 25-7 and 25-8).15
History
When tricuspid atresia occurs with normally related great arteries, males and females are equally represented,15 but when the great arteries are transposed, males predominate15 unless there is juxtaposition of the atrial appendages.31 Tricuspid atresia has been reported in siblings,35–37 in families,38 and in experimental animals.39
In about 6% of infants with tricuspid atresia, birth is premature.5 Survival depends on an adequate interatrial communication and adequate regulation of pulmonary blood flow.15,23 Increased longevity incurs the risk of infective endocarditis, paradoxical emboli, and brain abcess.15,22,40 Life span is less than 6 months when tricuspid atresia occurs with normally related great arteries and pulmonary atresia (see Figure 25-2A), but exceptional survivals have been recorded at age 21 years41 and 22 years.42 Acquired pulmonary atresia takes the form of spontaneous closure of the ventricular septal defect (see previous), an eventuality that usually occurs in the first year of life.15,19 Survival then depends on patency of the ductus arteriosus, an advantage that is seldom realized. Exceptional survivals have nevertheless been reported at age 8 years (see Figure 25-1), 18 years,15 and 27 years,20 in addition to a 21-year-old woman who survived because adequate pulmonary blood flow was achieved by an anomalous artery connecting the ascending aorta to the pulmonary trunk.3 The most exceptional survival was a 65-year-old man with tricuspid atresia, pulmonary atresia, an ostium secundum atrial septal defect, and large aortic-to-pulmonary arterial collaterals.43
Most patients with tricuspid atresia and normally related great arteries die in the first year because an already restrictive ventricular septal defect decreases in size or closes altogether.15 When the ventricular septal defect adequately regulates pulmonary blood flow, survivals have been realized from the second into the fifth decade.44,45 Two patients lived to 57 years of age,23 and a 30-year-old woman had a relatively uneventful pregnancy and a dysmature but otherwise healthy offspring.46 If the ventricular septal defect is nonrestrictive (see Figure 25-2C), increased pulmonary blood flow results in excessive volume overload of the left ventricle and congestive heart failure. However, one such patient was alive at age 6 years (see Figure 25-5), and survivals have been reported to age 32 years and 45 years,40,47 with an exceptional survival to age 57 years.48
The same longevity patterns occur with tricuspid atresia, complete transposition of the great arteries, and a nonrestrictive ventricular septal defect (see Figure 25-3C) in which regulation of pulmonary blood flow depends on pulmonary vascular resistance (see Figure 25-8). Exceptional survivals have been reported to the mid and late teens.15,49 Satisfactory regulation of pulmonary blood flow is more likely to be achieved by pulmonary stenosis (see Figure 25-3B)15 with which isolated patients have lived into the second, third, and fourth decades,21,23,40 with one patient dying at age 56 years.50
Survival in congenital tricuspid stenosis depends on the degree of obstruction and on an adequate interatrial communication.51,52 A 20-year-old woman was acyanotic (see Figure 25-11),52 a cyanotic patient underwent surgical repair at age 50 years (see Figure 25-4), and a cyanotic man survived to age 57 years.51
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree